Endpoints for Transcatheter Mitral Treatment Is A Hard













- Slides: 27

Endpoints for Transcatheter Mitral Treatment: Is A Hard Primary Endpoint Still Necessary? Ori Ben-Yehuda, MD Clinical Trials Center Cardiovascular Research Foundation & Columbia University New York, NY

Ori Ben-Yehuda, MD- Disclosures Consultant to Cardiovalve, Inc.

FMR: More complicated than AS

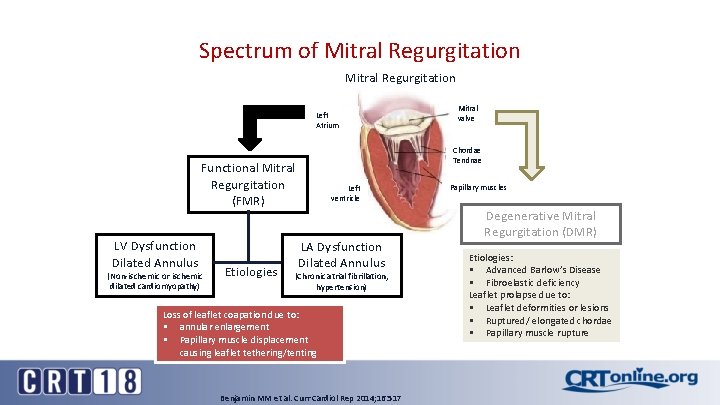
Spectrum of Mitral Regurgitation Left Atrium Chordae Tendnae Functional Mitral Regurgitation (FMR) LV Dysfunction Dilated Annulus (Non-ischemic or ischemic dilated cardiomyopathy) Etiologies Mitral valve Left ventricle LA Dysfunction Dilated Annulus (Chronic atrial fibrillation, hypertension) Loss of leaflet coapation due to: § annular enlargement § Papillary muscle displacement causing leaflet tethering/tenting Benjamin MM et al. Curr Cardiol Rep 2014; 16: 517 Papillary muscles Degenerative Mitral Regurgitation (DMR) Etiologies: § Advanced Barlow’s Disease § Fibroelastic deficiency Leaflet prolapse due to: § Leaflet deformities or lesions § Ruptured/ elongated chordae § Papillary muscle rupture

Functional MR: Role of Tethering Levine et al. Curr Cardiol Rep 2002; 4: 125 -9

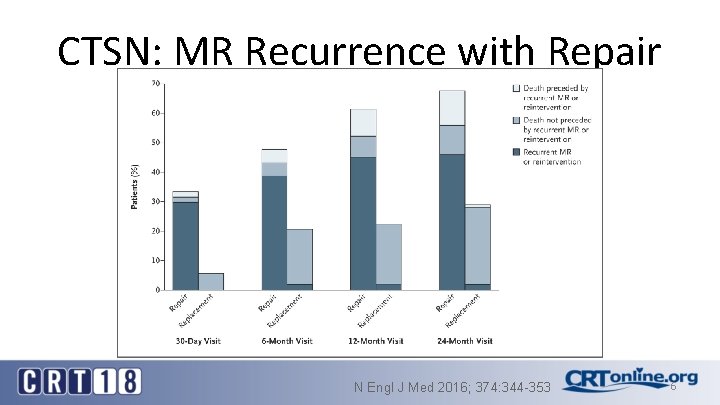
CTSN: MR Recurrence with Repair N Engl J Med 2016; 374: 344 -353 6

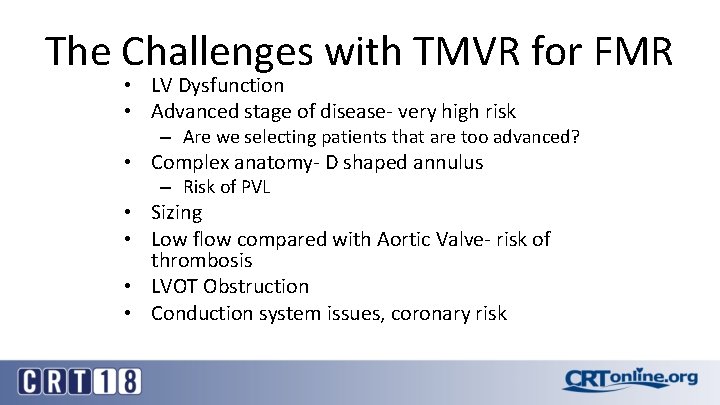
The Challenges with TMVR for FMR • LV Dysfunction • Advanced stage of disease- very high risk – Are we selecting patients that are too advanced? • Complex anatomy- D shaped annulus – Risk of PVL • Sizing • Low flow compared with Aortic Valve- risk of thrombosis • LVOT Obstruction • Conduction system issues, coronary risk

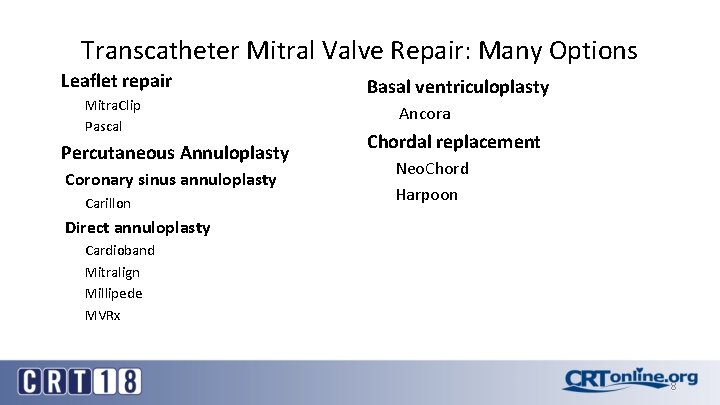
Transcatheter Mitral Valve Repair: Many Options Leaflet repair Mitra. Clip Pascal Percutaneous Annuloplasty Coronary sinus annuloplasty Carillon Basal ventriculoplasty Ancora Chordal replacement Neo. Chord Harpoon Direct annuloplasty Cardioband Mitralign Millipede MVRx 8

COAPT Trial: Design ~610 patients enrolled at up to 100 sites Symptomatic HF treated with maximally tolerated guideline directed medical therapy Significant FMR (≥ 3+ by echo core lab) Not appropriate for MV surgery as determined by site’s local heart team Valve anatomy eligible for Mitra. Clip treatment Randomize 1: 1 Control group Standard of care N~305 Mitra. Clip N~305 Clinical and TTE follow-up: Baseline, treatment, 1 -week (phone), 1, 6, 12, 18, 24, 36, 48, 60 months Primary endpoint: Hospitalization for heart failure within 2 years Principal Investigators: Gregg Stone, Michael Mack Heart Failure Co-Principal Investigators: William Abraham, Jo. Ann Lindenfeld Sponsor: Abbott Vascular

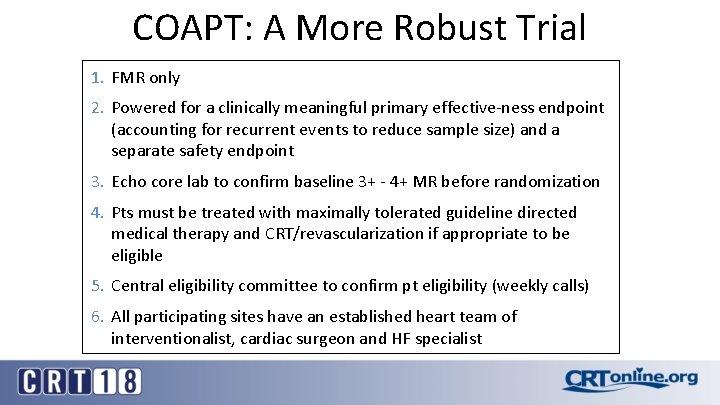
COAPT: A More Robust Trial 1. FMR only 2. Powered for a clinically meaningful primary effective-ness endpoint (accounting for recurrent events to reduce sample size) and a separate safety endpoint 3. Echo core lab to confirm baseline 3+ - 4+ MR before randomization 4. Pts must be treated with maximally tolerated guideline directed medical therapy and CRT/revascularization if appropriate to be eligible 5. Central eligibility committee to confirm pt eligibility (weekly calls) 6. All participating sites have an established heart team of interventionalist, cardiac surgeon and HF specialist

COAPT: Enrollment Between December 2012 and October 2016, 482 patients have been randomized at 84 active sites ~0. 15 pts/site/month Enrollment of 610 pts took 4. 5 years after initiation

COAPT: Why so slow? • Multiple protocol versions and submissions (to sites and FDA) • Currently on version 8. 0 • Evolved from a HF trial to a high-risk MV surgery trial back to a HF trial • Sample size increased to account for competing risk of death • • • Contracting/reimbursement at many sites is difficult Some CMS MAC approvals have been difficult Numerous and complex inclusion and exclusion criteria Screening processes are intense Eligibility call requirements are challenging (weekly call) • Expert committee: Physician moderator (non-voting), 2 HF specialists and 2 cardiac surgeons • Site: Local PI/HF physician(s) and surgeon to declare that he/she won’t operate • We are declining/deferring a significant minority - in particular, GDMT criteria are not easy to meet • HF/FMR pts are de-centralized – varies at each hospital

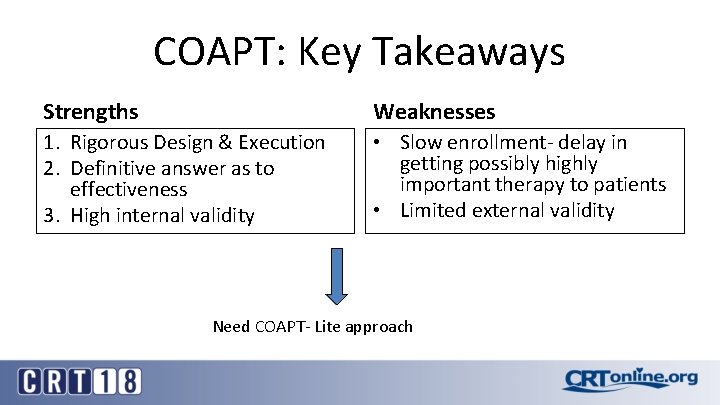
COAPT: Key Takeaways Strengths Weaknesses 1. Rigorous Design & Execution 2. Definitive answer as to effectiveness 3. High internal validity • Slow enrollment- delay in getting possibly highly important therapy to patients • Limited external validity Need COAPT- Lite approach

Future Directions: “COAPT-Lite” • Relaxation of GDMT criteria to increase enrollment • Use of surrogate endpoints- as part of composite • Primary endpoint at 1 year- continue to follow cohort for harder endpoints (eg: hospitalizations) • Co-primary of MR reduction

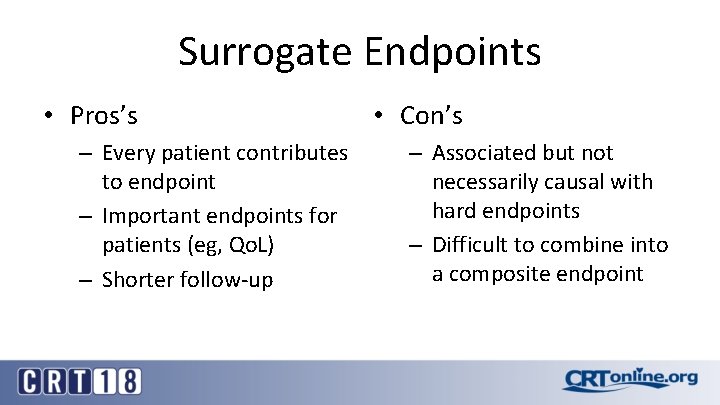
Surrogate Endpoints • Pros’s – Every patient contributes to endpoint – Important endpoints for patients (eg, Qo. L) – Shorter follow-up • Con’s – Associated but not necessarily causal with hard endpoints – Difficult to combine into a composite endpoint

Type of Surrogate Endpoints Applicable to CV Devices • Imaging – Echo- Reduction in stenosis, valve regurgitation, etc. , EF – MRI- Reduction in LVH, Infarct Size • Exercise endpoints – 6 MWT, ETT, CPET • Quality of Life – Generic- SF-36/SF-12, EQ-5 – Disease specific- SAQ, MLWHFQ, KCCQ • Biomarkers – NT-NBP, STS

Traditional tests of exercise tolerance in HF clinical trials • Six minute walk test (6 MWT) – Indicator of sub-maximal exercise capacity, prognostic (PH, LVSD) – Volitional, may be reflective of maximal exercise capacity and source of limitation remains unknown • Cardiopulmonary Exercise Test (CPET) – Gas exchange patterns characteristic of diseases that provide insight into the organ system limiting exercise capacity • • • Peak VO 2 Ventilatory Anaerobic Threshold VE/VCO 2 slope

Virtues of CPET/Peak VO 2 • Peak VO 2 is the gold standard measure of cardiopulmonary function • More precise and reproducible than other measures of physical function • Peak VO 2 is responsive to therapy (drug, device, exercise training) • Peak VO 2 and other CPET responses strongly associated with clinical outcomes • Improvement in hardware/software

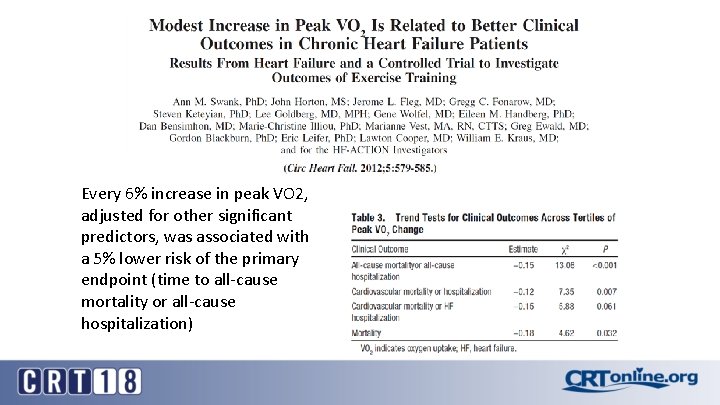
Every 6% increase in peak VO 2, adjusted for other significant predictors, was associated with a 5% lower risk of the primary endpoint (time to all-cause mortality or all-cause hospitalization)

6 Minute Walk • Primary measure is total distance walked – Performed in a hallway – Patient is permitted to take breaks, but timer continues • Measure of exercise tolerance – Most useful among patients who: • have limited exercise tolerance • are limited by symptoms (eg, shortness of breath)

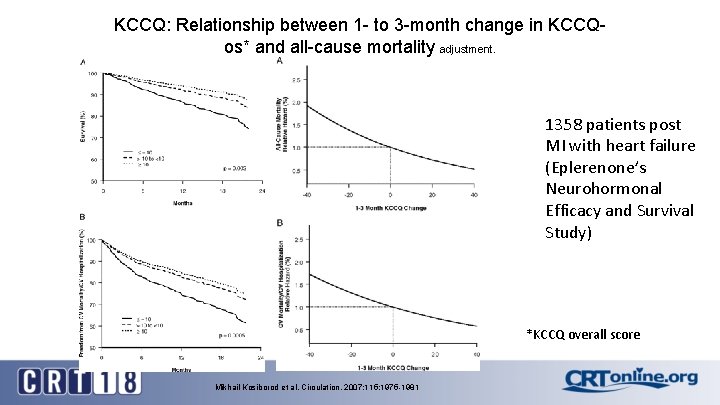
KCCQ: Relationship between 1 - to 3 -month change in KCCQos* and all-cause mortality adjustment. 1358 patients post MI with heart failure (Eplerenone’s Neurohormonal Efficacy and Survival Study) *KCCQ overall score Mikhail Kosiborod et al. Circulation. 2007; 115: 1975 -1981

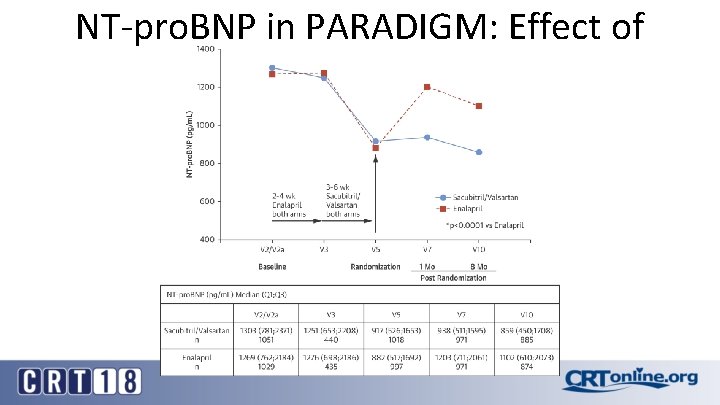
NT-pro. BNP in PARADIGM

NT-pro. BNP in PARADIGM: Effect of Treatment

Integrating Surrogates into Composite Endpoints • Composite endpoints are problematic- even for hard endpoints – eg: Death, Stroke, MI; mortality and hospitalization • Even more challenging for surrogate endpoints, esp in combinationwith hardpoints – eg: hospitalization plus improvement in KCCQ! How does one combine into a single endpoint?

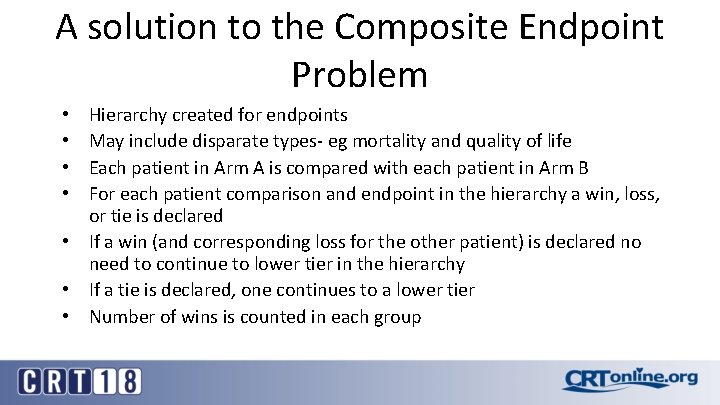
A solution to the Composite Endpoint Problem Hierarchy created for endpoints May include disparate types- eg mortality and quality of life Each patient in Arm A is compared with each patient in Arm B For each patient comparison and endpoint in the hierarchy a win, loss, or tie is declared • If a win (and corresponding loss for the other patient) is declared no need to continue to lower tier in the hierarchy • If a tie is declared, one continues to a lower tier • Number of wins is counted in each group • •

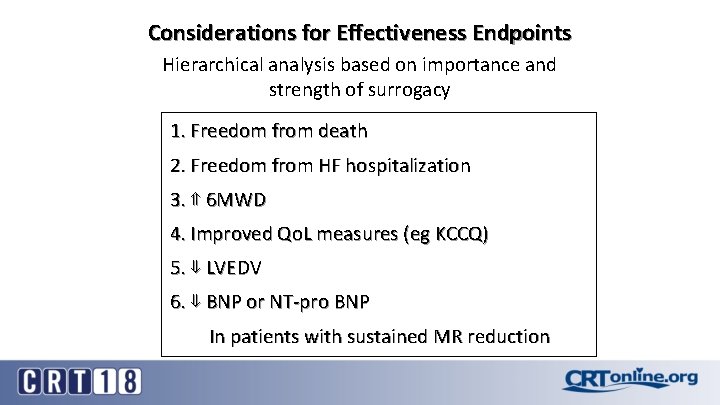
Considerations for Effectiveness Endpoints Hierarchical analysis based on importance and strength of surrogacy 1. Freedom from death 2. Freedom from HF hospitalization 3. ⇑ 6 MWD 4. Improved Qo. L measures (eg KCCQ) 5. ⇓ LVEDV 6. ⇓ BNP or NT-pro BNP In patients with sustained MR reduction

New Mitral Annuloplasty Devices with IDE Trials Population N Randomized Primary EP Cardioband Carillon Symptomatic HF and FMR Ischemic or non-ischemic cardiomyopathy; optimized and stable medical HF regimen ~400 2: 1 1 year: Prevalence of MR ≤ 2+ and 1 -year efficacy: Clinical endpoints hierarchical comparison of alland regurgitant volume cause mortality, # of HF hospitalizations, 6 minute walk test 1 -year safety: Device-related major adverse events and KCCQ clinicaltrials. gov