Endoscopic grading of gastric intestinal metaplasia EGGIM 2020





















- Slides: 32

Endoscopic grading of gastric intestinal metaplasia (EGGIM) 2020 -10 -08 R 3 이기환 / Pf. 정현수

Introduction • The gastric precancerous cascade

Introduction • The updated Sydney system

Introduction • The OLGA & OLGIM staging system

Introduction • The OLGA & OLGIM staging system

Introduction

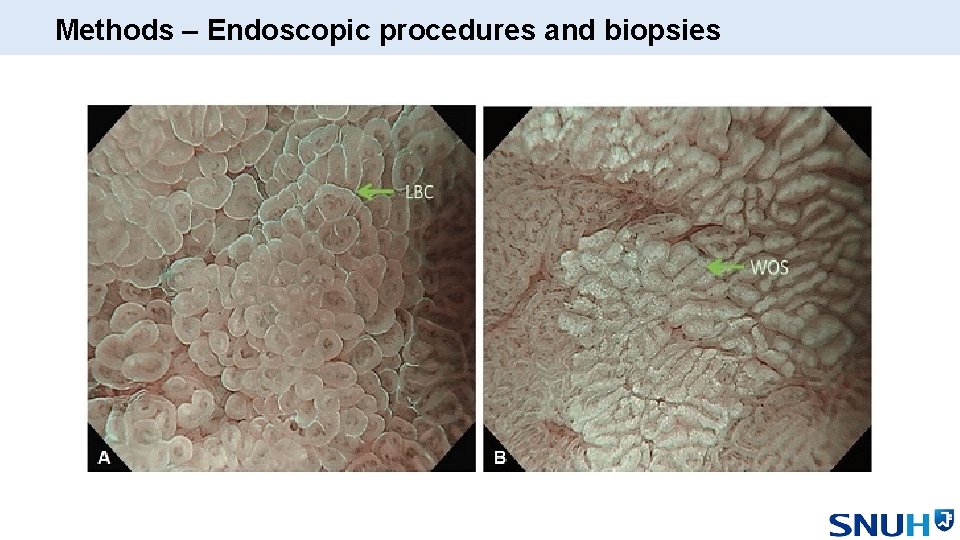
Methods – Endoscopic procedures and biopsies

Introduction • The EGGIM staging system

Subarticle

Background • EGGIM may be used to assess a patient’s risk • Diagnostic accuracy of EGGIM compared with OLGIM • 98% among 201 patients when assessed solely by a single endoscopist → Aim : To formally validate the diagnostic accuracy of EGGIM • using OLGIM as the reference test • in a prospective multicenter study

Methods – Study design and participants • Prospective study involving two endoscopic academic centers (Italy & Portugal) • From January 2016 to September 2017 • Consecutive outpatients undergoing gastroscopy with HR-NBI gastroscopes (GIFH 185 or GIF-HQ 190; Olympus) • Exclusion criteria • Known OLGIM • Contraindication for biopsies • Significant comorbidities • Previous gastric neoplasia or surgery • Intolerance of endoscopic procedure

Methods – Endoscopic procedures and biopsies • Fully trained endoscopists with NBI experience • Interobserver agreement was 0. 93 for the first 20 procedures • Detailed observation of the gastric mucosa with HR-WLE • HR-NBI observation of the entire gastric mucosa of the antrum, angle & body • Suspicious areas were targeted for biopsy • Random biopsies were taken if GIM was not suspected • Two expert GI pathologists evaluated specimen

Methods – Endoscopic procedures and biopsies

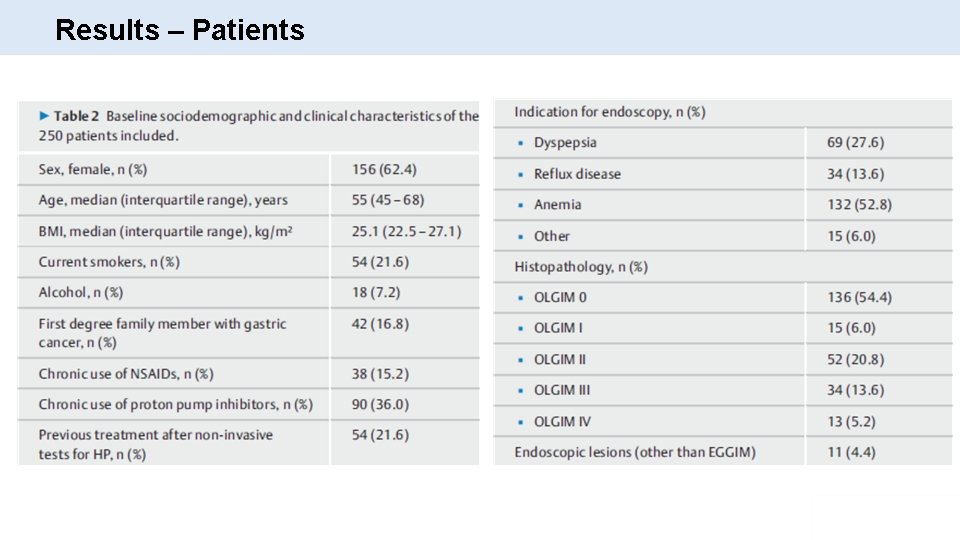
Results – Patients

Results EGGIM score Patient number 0 128 (51. 2%) 1 0 (0%) 2 16 (6. 4%) 3 7 (2. 8%) 4 46 (18. 4%) 5 9 (3. 6%) 6 18 (7. 2%) 7 11 (4. 4%) 8 9 (3. 6%) 9 2 (0. 8%) 10 4 (1. 6%)

Results • EGGIM scores compared to OLGIM stage III & IV AUC = 0. 96

Results

Results

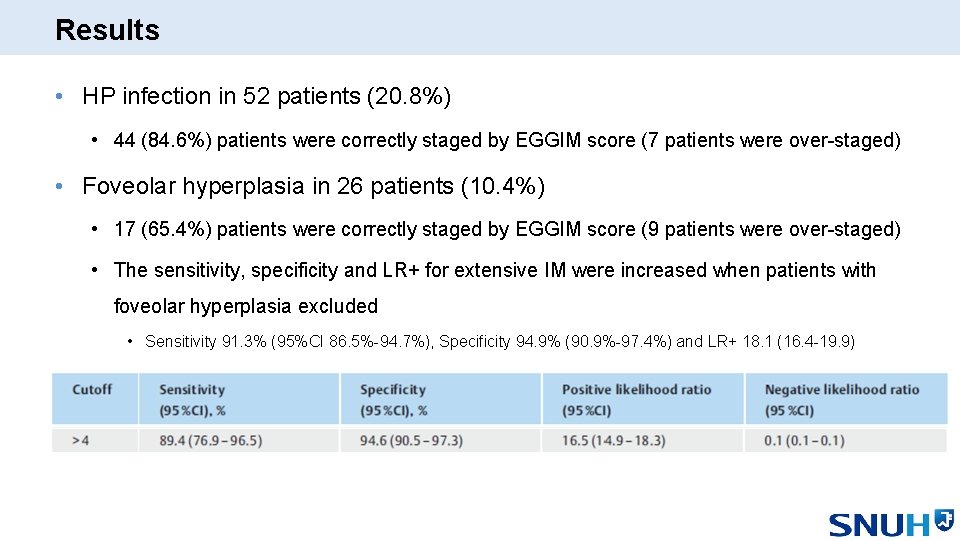
Results • HP infection in 52 patients (20. 8%) • 44 (84. 6%) patients were correctly staged by EGGIM score (7 patients were over-staged) • Foveolar hyperplasia in 26 patients (10. 4%) • 17 (65. 4%) patients were correctly staged by EGGIM score (9 patients were over-staged) • The sensitivity, specificity and LR+ for extensive IM were increased when patients with foveolar hyperplasia excluded • Sensitivity 91. 3% (95%CI 86. 5%-94. 7%), Specificity 94. 9% (90. 9%-97. 4%) and LR+ 18. 1 (16. 4 -19. 9)

Discussion • First prospective multicenter study of EGGIM compared to OLGIM. • EGGIM could be used to avoid biopsies. • Foveolar hyperplasia might be a possible confounding factor.

Main article

Background • OLGIM systems are reliable predictors of the risk for gastric cancer. • EGGIM was validated in individuals without gastric cancer using histology as gold standard. • Strong correlation between EGGIM and OLGIM stages. • The independent value of EGGIM stages for risk assessment was not proven. → Aim : To assess the value of EGGIM, OLGA, & OLGIM for EGN

Method – Study design and patient selection • Cases - 187 cases / 250 eligible cases • 280 patients who underwent ESD for early gastric epithelial neoplasia • Exclusion criteria (n=30) • ESD for synchronous, ES metachronous or recurrent lesion (n=24), Advanced lesion (n=1), Past gastric surgery (n=2), Hereditary gastrointestinal syndrome (n=3) • Controls – 187 cases / 281 eligible cases • 483 non-repeated subjects who underwent EGD with gastric biopsies for staging of gastritis • Exclusion criteria (n=202) • Previous or current gastric lesion (n=100), past gastric surgery (n=2), hereditary gastrointestinal syndrome (n=99), non-available EGD report (n=1)

Method – Data collection • Medical records from 2012 to 2017 • Family history of GC • Smoking status • Alcohol consumption • Use of aspirin or PPI • H. pylori infection status • EGGIM, OLGA, OLGIM classification

Results • EGN group (n=187) • 90 (48%) intraepithelial neoplasm • 13 (7%) low-grade dysplastic lesions • 77 (41%) high-grade dysplastic lesions • 97 (52%) early GCs • 74 (40%) p. T 1 a, 23 (12%) p. T 1 b • 90 (93%) WD/MD, 7 (7%) PD or PCC

Results

Results • EGGIM, OLGA, OLGIM scores compared with presence of EGN EGGIM AUC 0. 84 (95% CI 0. 70 to 0. 99) OLGA AUC 0. 80 (95% CI 0. 68 to 0. 92) OLGIM AUC 0. 84 (95% CI 0. 71 to 0. 98)


Discussion • First study to show that EGGIM can be useful for risk assessment of EGN. • Only included patients with EGNs resected endoscopically. • More legitimate for the intestinal type of GC. • OLGA stage I/II was not significantly associated with the risk of EGN, where as OLGIM I/II was. • EGGIM is only studied and validated for NBI. • Extension to other providers needs to be investigated.


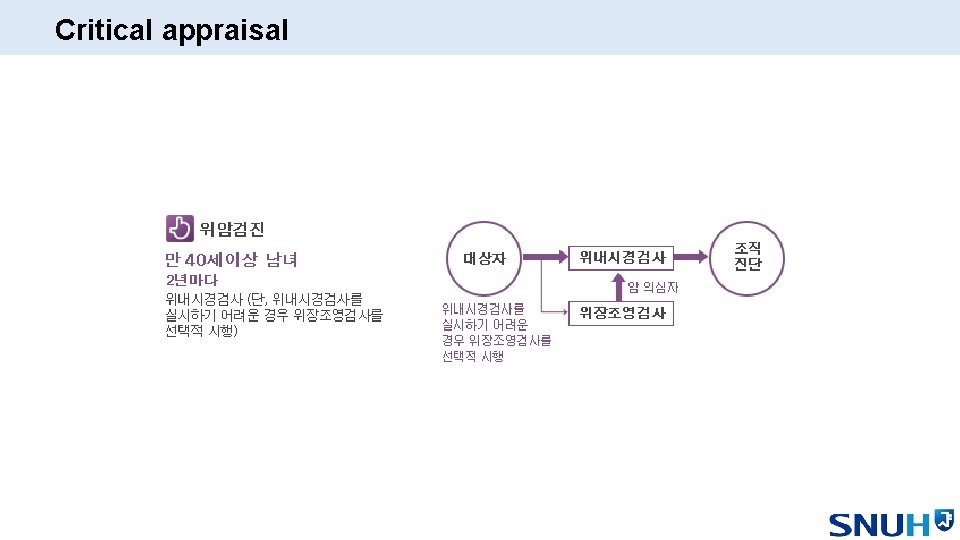
Critical appraisal
