Endocrinology MODES OF HORMONE DELIVERY I n ENDOCRINE


































































































- Slides: 143

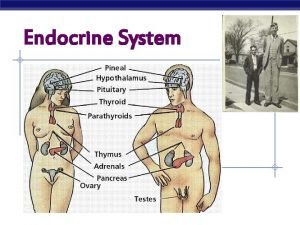
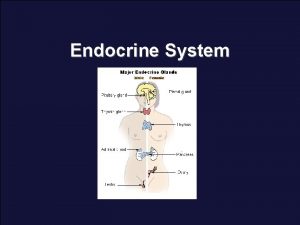
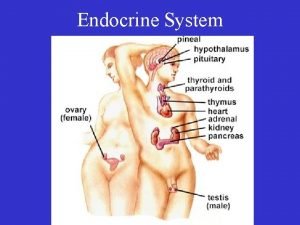
Endocrinology


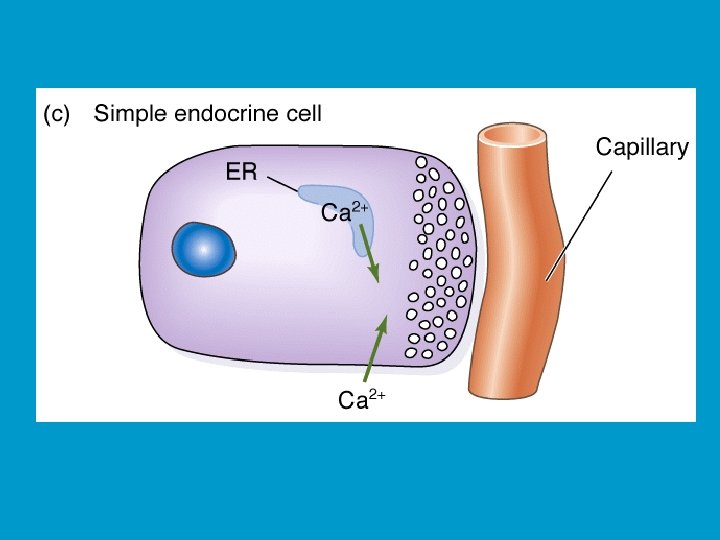
MODES OF HORMONE DELIVERY I: n ENDOCRINE: n n Most common (classical) mode, hormones delivered to target cells by blood. PARACRINE: Hormone released diffuses to its target cells through immediate extracellular space. n Blood is not directly involved in the delivery. n

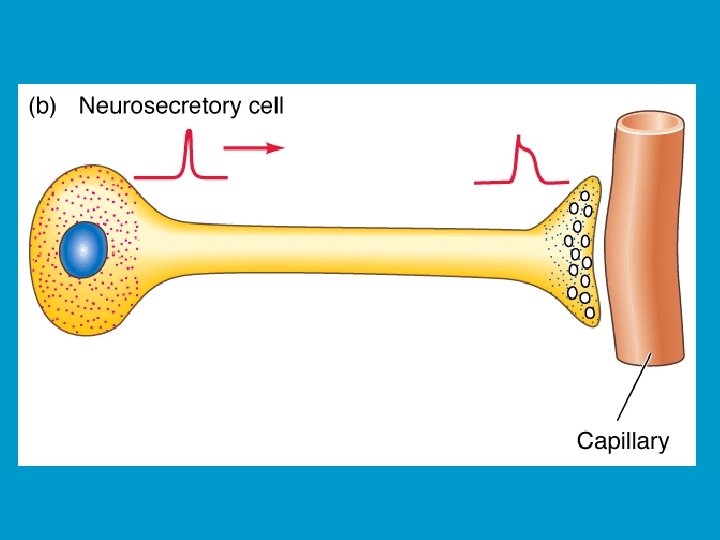
MODES OF HORMONE DELIVERY II: n NEUROENDOCRINE: n n Hormone is produced and released by a neuron, delivered to target cells by blood. AUTOCRINE: n Hormone released feeds-back on the cell of origin, again without entering blood circulation.

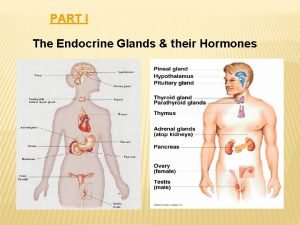
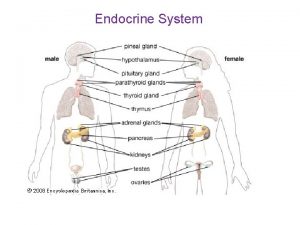
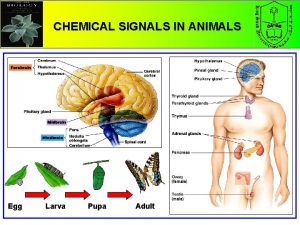
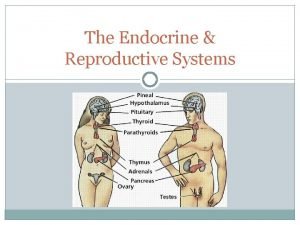
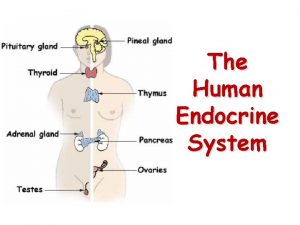
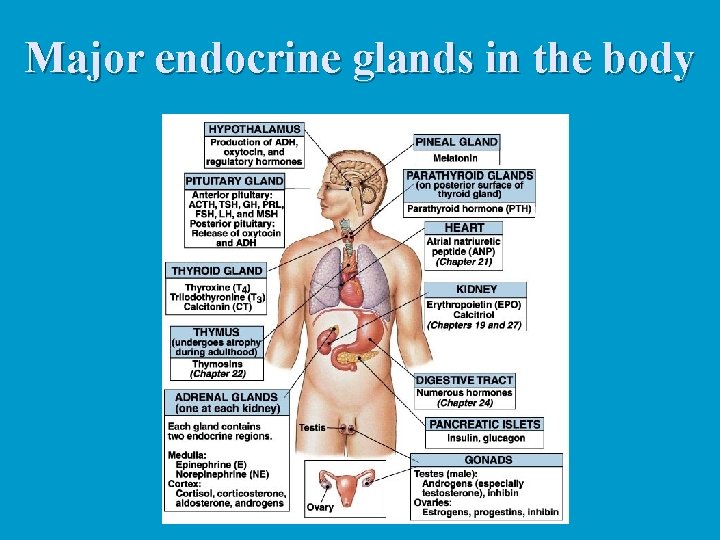
Major endocrine glands in the body

HORMONE-TARGET CELL SPECIFICITY n Only target cells, or cells that have specific receptors, will respond to the hormone’s presence. n The strength of this response will depend on: n Blood levels of the hormone n The relative numbers of receptors for that hormone on or in the target cells n The affinity (or strength of interactions) of the hormone and the receptor.

HALF-LIFE, ONSET, and DURATION of HORMONE ACTIVITY The affinity of hormones to their specific receptors is typically very high n The actual concentration of a circulating hormone in blood at any time reflects: n Its rate of release. n The speed of its inactivation and removal from the body. n

n The half-life is the time required for the hormone to loose half of its original effectiveness (or drop to half of its original concentration. n The time required for hormone effects to take place varies greatly, from almost immediate responses to hours or even days. n In addition, some hormones are produced in an inactive form and must be activated in the target cells before exerting cellular responses. n In terms of the duration of hormone action, it ranges from about 20 minutes to several hours, depending on the hormone.

CONTROL OF HORMONE RELEASE: n n n The synthesis and secretion of most hormones are usually regulated by negative feedback systems. As hormone levels rise, they stimulate target organ responses. These in turn, inhibit further hormone release. The stimuli that induce endocrine glands to synthesize and release hormones belong to one of the following major types: n n n Humoral Neural Hormonal

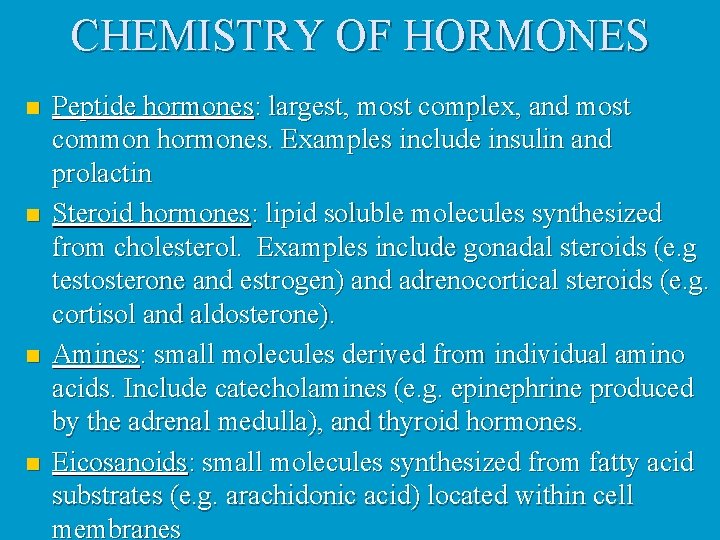
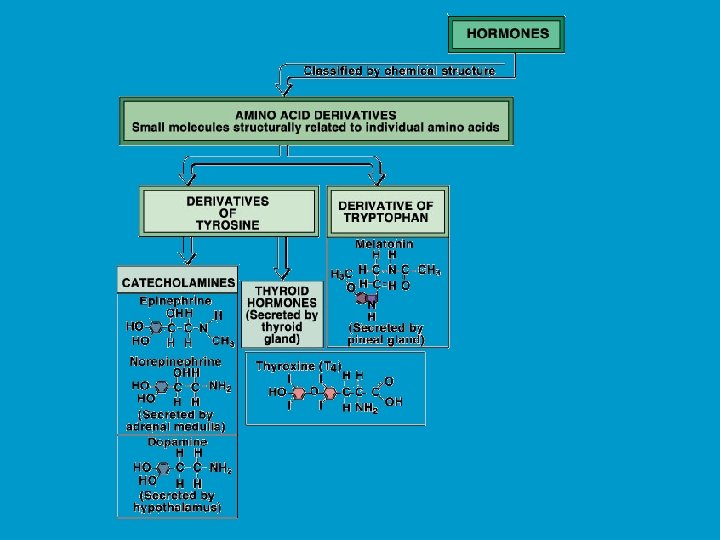
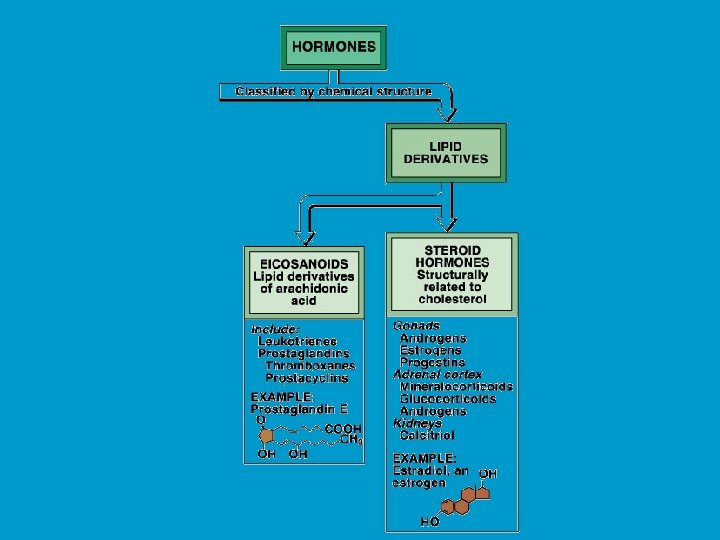
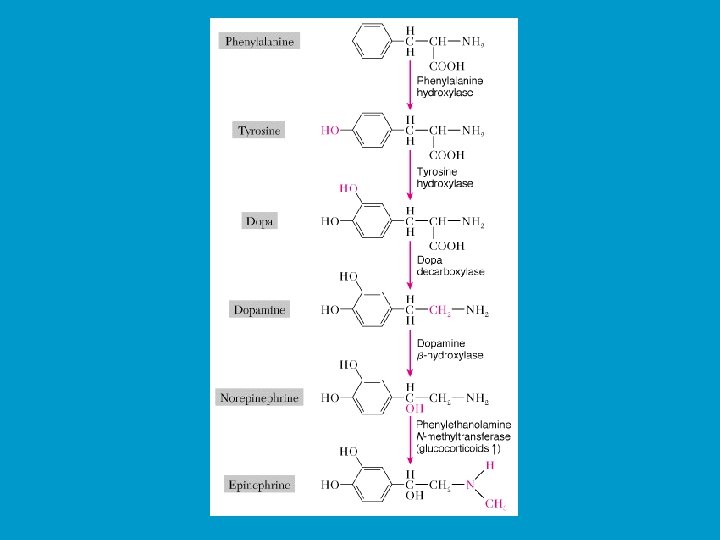
CHEMISTRY OF HORMONES n n Peptide hormones: largest, most complex, and most common hormones. Examples include insulin and prolactin Steroid hormones: lipid soluble molecules synthesized from cholesterol. Examples include gonadal steroids (e. g testosterone and estrogen) and adrenocortical steroids (e. g. cortisol and aldosterone). Amines: small molecules derived from individual amino acids. Include catecholamines (e. g. epinephrine produced by the adrenal medulla), and thyroid hormones. Eicosanoids: small molecules synthesized from fatty acid substrates (e. g. arachidonic acid) located within cell membranes





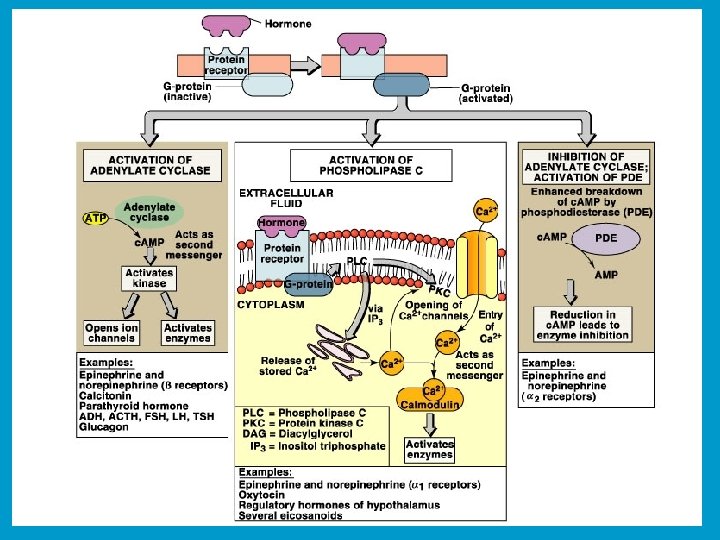
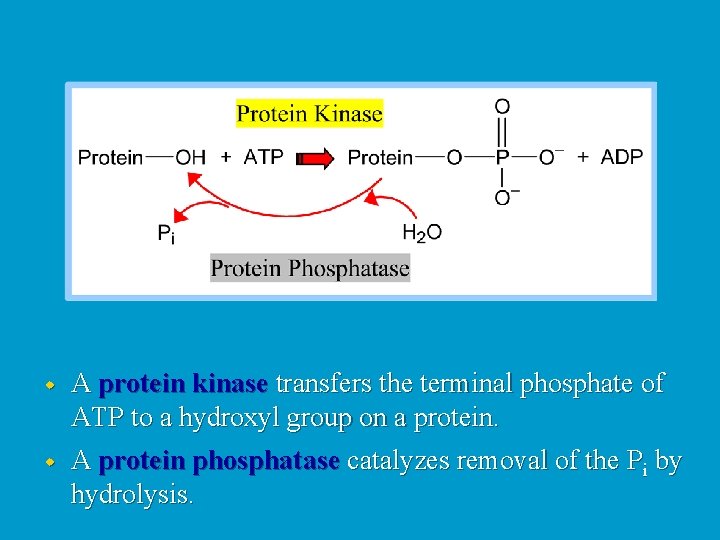
w A protein kinase transfers the terminal phosphate of ATP to a hydroxyl group on a protein. w A protein phosphatase catalyzes removal of the Pi by hydrolysis.

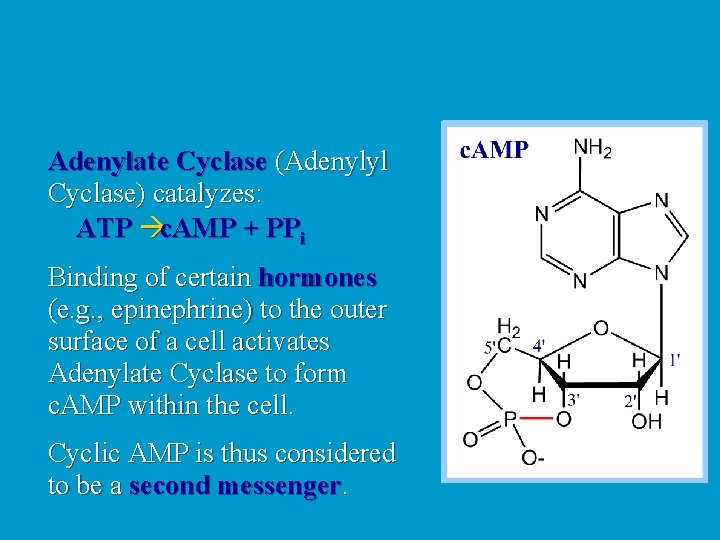
Adenylate Cyclase (Adenylyl Cyclase) catalyzes: ATP c. AMP + PPi Binding of certain hormones (e. g. , epinephrine) to the outer surface of a cell activates Adenylate Cyclase to form c. AMP within the cell. Cyclic AMP is thus considered to be a second messenger.

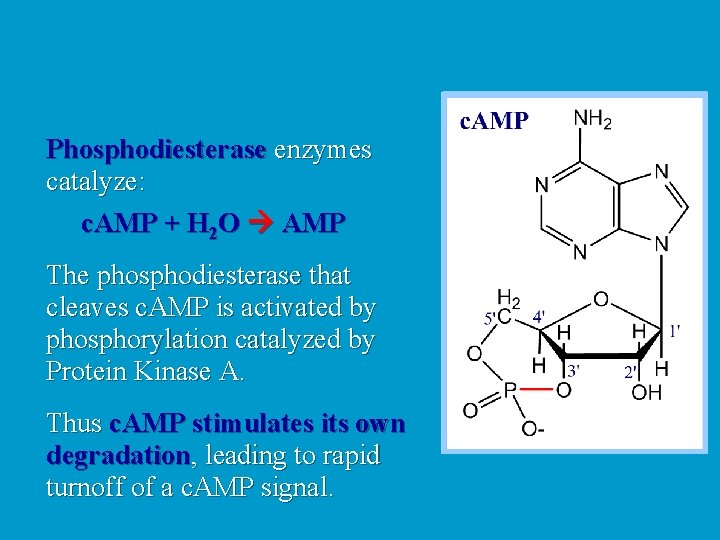
Phosphodiesterase enzymes catalyze: c. AMP + H 2 O AMP The phosphodiesterase that cleaves c. AMP is activated by phosphorylation catalyzed by Protein Kinase A. Thus c. AMP stimulates its own degradation, leading to rapid turnoff of a c. AMP signal.

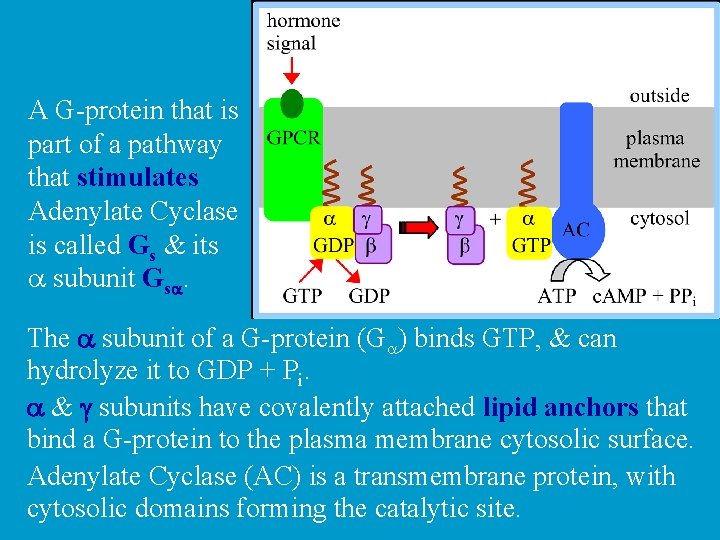
A G-protein that is part of a pathway that stimulates Adenylate Cyclase is called Gs & its a subunit Gsa. The a subunit of a G-protein (Ga) binds GTP, & can hydrolyze it to GDP + Pi. a & g subunits have covalently attached lipid anchors that bind a G-protein to the plasma membrane cytosolic surface. Adenylate Cyclase (AC) is a transmembrane protein, with cytosolic domains forming the catalytic site.

The complex of b & g subunits Gb, g inhibits Ga. The sequence of events by which a hormone activates c. AMP signaling: 1. Initially Ga has bound GDP, and a, b, & g subunits are complexed together.

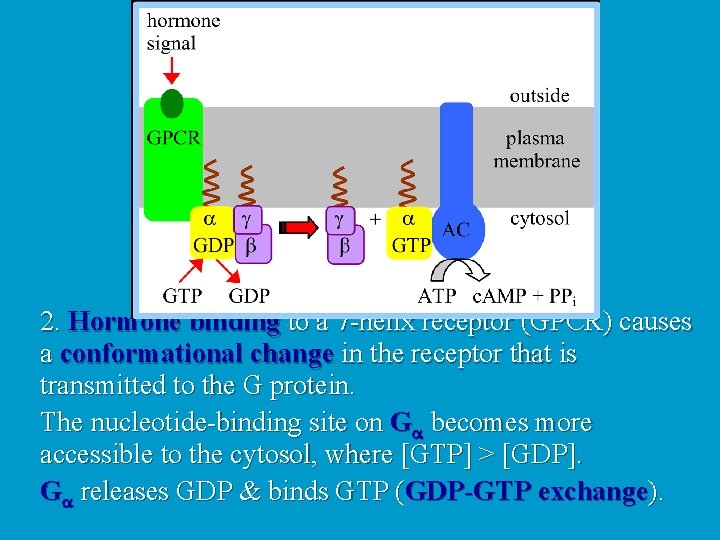
2. Hormone binding to a 7 -helix receptor (GPCR) causes a conformational change in the receptor that is transmitted to the G protein. The nucleotide-binding site on Ga becomes more accessible to the cytosol, where [GTP] > [GDP]. Ga releases GDP & binds GTP (GDP-GTP exchange).

3. Substitution of GTP for GDP causes another conformational change in Ga. Ga-GTP dissociates from the inhibitory bg complex & can now bind to and activate Adenylate Cyclase.

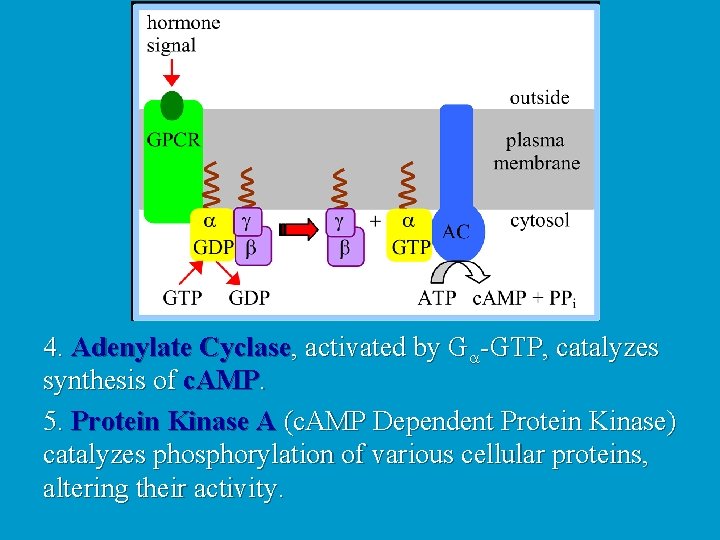
4. Adenylate Cyclase, activated by Ga-GTP, catalyzes synthesis of c. AMP. 5. Protein Kinase A (c. AMP Dependent Protein Kinase) catalyzes phosphorylation of various cellular proteins, altering their activity.

Turn off of the signal: 1. Ga hydrolyzes GTP to GDP + Pi. (GTPase). The presence of GDP on Ga causes it to rebind to the inhibitory bg complex. Adenylate Cyclase is no longer activated. 2. Phosphodiesterase catalyzes hydrolysis of c. AMP.

Turn off of the signal (cont. ): 3. Hormone receptor desensitization occurs. This process varies with the hormone. w Some receptors are phosphorylated via G-proteincoupled receptor kinases. w The phosphorylated receptor may then bind to a protein arrestin that blocks receptor-G-protein activation & promotes removal of the receptor from the membrane by clathrin-mediated endocytosis. 4. Protein Phosphatase catalyzes removal by hydrolysis of phosphates that were attached to proteins via Protein Kinase A.


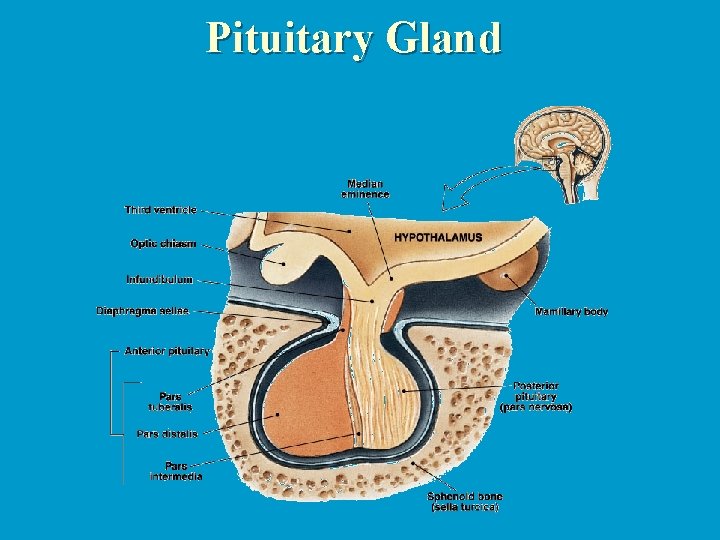
Pituitary Gland


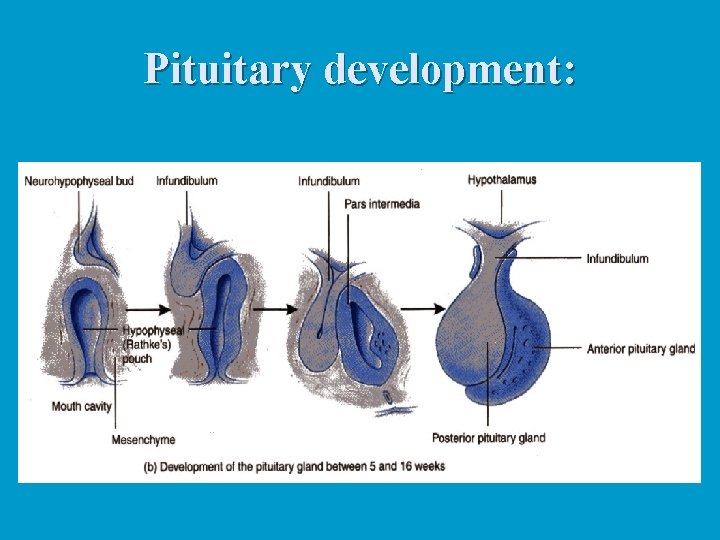
Pituitary development:









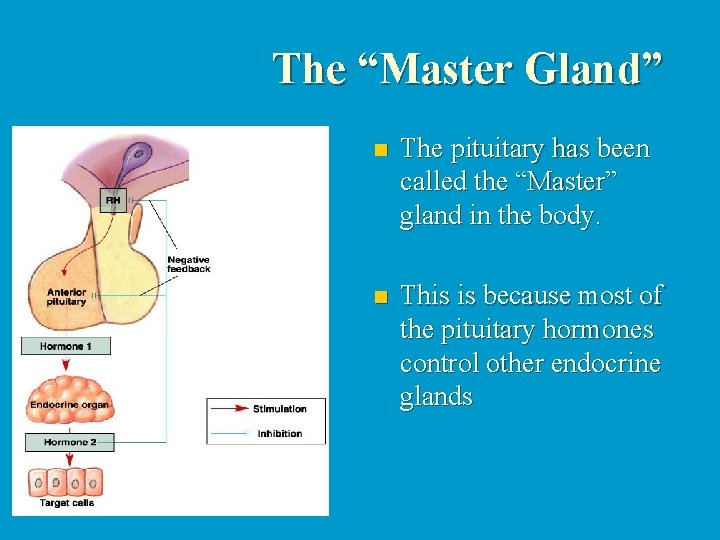
The “Master Gland” n The pituitary has been called the “Master” gland in the body. n This is because most of the pituitary hormones control other endocrine glands

Hormones of the anterior pituitary n There are 6 main hormones which are secreted by the adenohypophysis: n 1) Growth hormone (also known as somatotropin). n 2) Thyroid-stimulating hormone (also known as thyrotropin). n 3) Adrenocorticotropic hormone (also known as corticotropin). n 4) Prolactin. n 5) Follicle-stimulating hormone. n 6) Luteinizing hormone.

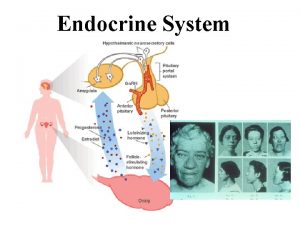
Control of pituitary gland secretion Secretion of each hormone by the adenohypophysis is controlled by neurohormones secreted by nerves in the hypothalamus. n In most cases there are two neurohormones controlling the secretion of a pituitary hormone. One which stimulates pituitary secretion and one which inhibits pituitary secretion. n


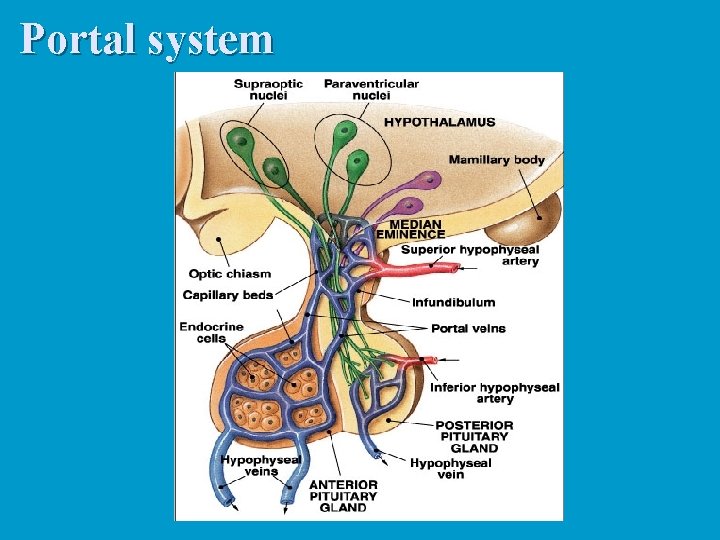
Neurohormones: Are hormones secreted by nerve cells. These are true hormones, since they are secreted into the bloodstream. n All are secreted by neurosecretory neurons in the hypothalamus. n They are secreted into the hypophyseal portal system, which then carries the blood to the anterior pituitary. n

Pituitary portal system n n n Arterioles break into capillaries in the hypothalamus. The axons of the neurosecretory cells form plexuses with these capillaries. Downstream, the capillaries combine into a vein which carries the blood to the pars distalis. The vein breaks into a capillary network which supplies all the cells of the anterior lobe. Thus, the neurohormones are carried directly (well, sort of) from the hypothalamus to the adenohypophysis.

Portal system

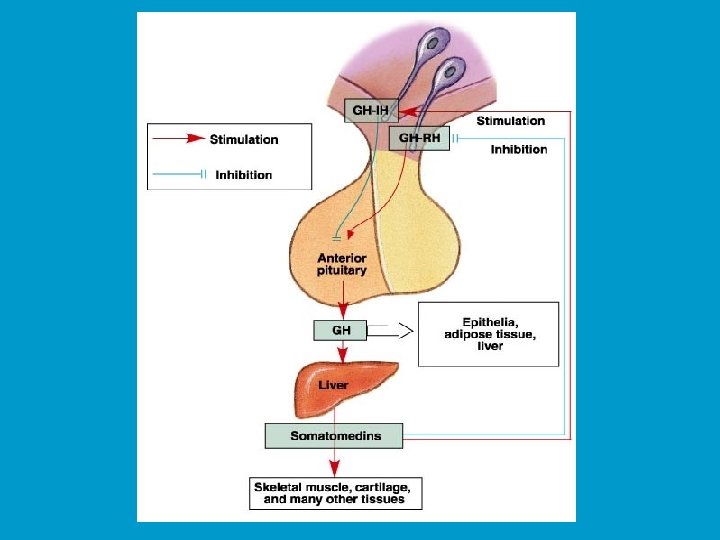
Growth hormone (GH) n Growth hormone is secreted by somatotrophs. n GH is a protein hormone consisting of a single peptide chain of 191 amino acids. n GH secretion is stimulated by the secretion of Growth Hormone Releasing Hormone (GHRH) by the hypothalamus. n GH secretion is inhibited by the secretion of somatostatin by the hypothalamus. n GH activates a tyrosine kinase receptor.

Functions of GH: n GH has effects of every cell of the body, either directly or indirectly. Primarily, it decreases the uptake and metabolism of glucose. (Elevates plasma glucose) n Increases the breakdown of fat. (Increases the blood levels of fatty acids) n Increases the uptake of amino acids from the blood and increases protein synthesis in cell. (Decreases plasma amino acids)

Actions of GH on specific cell types: n Muscle cells: Increases amino acid uptake n Increases protein synthesis n Decreases glucose uptake n Net result: Increased Lean body n mass

n Chondrocytes: n increases uptake of sulfur increases chondroitin sulfate production n increases DNA, RNA synthesis n increases Protein synthesis n increases Amino acid uptake n increases Collagen synthesis n increases Cell size and number n n Net result: Increased Linear growth

n Hepatocytes: n Stimulates the production of somatomedins by the liver. n These somatomedins directly regulate metabolic function in target cells. They are also called insulin-like growth factors, or IGFs.


n Adipocytes: Decreases glucose uptake n Increases lypolysis n Net result: Decreased n Adiposity

n Other cell types in general: Increased protein synthesis n Increased DNA, RNA synthesis n Increased cell size and number n n n Net result: Increased organ size Increased organ function

Other considerations: n GH has a short half-life of about 20 minutes. However, the IGFs are much longer lived (T 1/2 of about 20 hours).

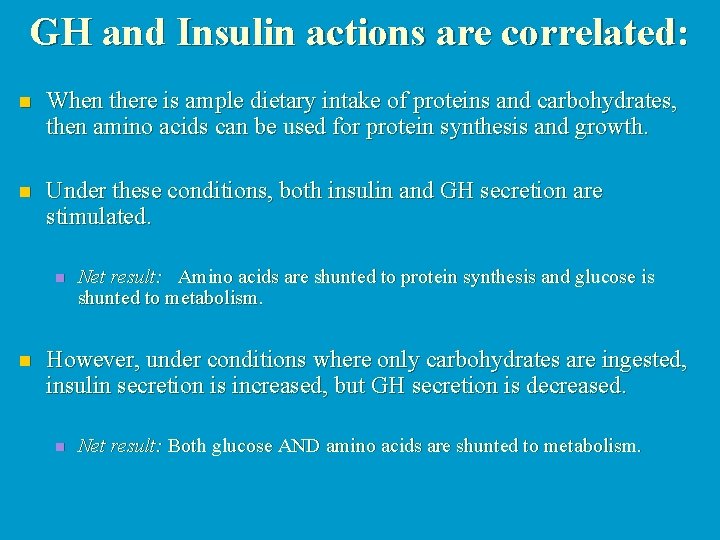
GH and Insulin actions are correlated: n When there is ample dietary intake of proteins and carbohydrates, then amino acids can be used for protein synthesis and growth. n Under these conditions, both insulin and GH secretion are stimulated. n n Net result: Amino acids are shunted to protein synthesis and glucose is shunted to metabolism. However, under conditions where only carbohydrates are ingested, insulin secretion is increased, but GH secretion is decreased. n Net result: Both glucose AND amino acids are shunted to metabolism.

Pathophysiology of abnormal GH secretion: n Hyposecretion: n Pre-adolescents: n n Decreased GH secretion (or sensitivity) results in slow growth and delayed onset of sexual maturation. These children also tend to be slightly chubby. Post-adolescents: n Generally, no serious problems are associated with hyposecretion of GH in mature individuals. However, in very severe cases there can be progeria (rapid and premature aging).

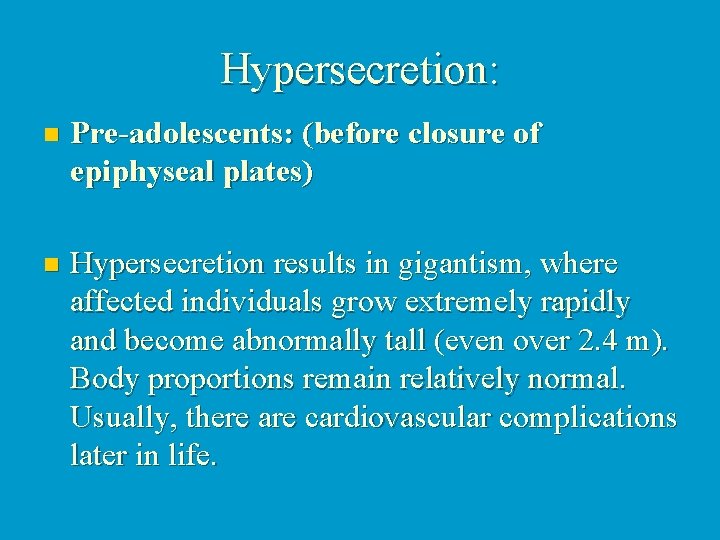
Hypersecretion: n Pre-adolescents: (before closure of epiphyseal plates) n Hypersecretion results in gigantism, where affected individuals grow extremely rapidly and become abnormally tall (even over 2. 4 m). Body proportions remain relatively normal. Usually, there are cardiovascular complications later in life.

n Post- adolescents: (after epiphyseal closure). n Hypersecretion results in tissue enlargement. This is particularly true of the bones, which get heavier and thicker. They cannot elongate since the epiphyseal plates are closed. A common symptom is a coarsening of the facial features and enlargement of the hands and feet. This condition is known as acromegaly.

Treatments of GH secretion disorders: n Hypersecretion is usually caused by a tumour in the pituitary gland. Treatment consists of surgical or radiation ablation of the tumour mass. n Hyposecretion is usually treated in children by hormone replacement therapy. This is generally not required in adults, unless GH secretion is completely abolished.


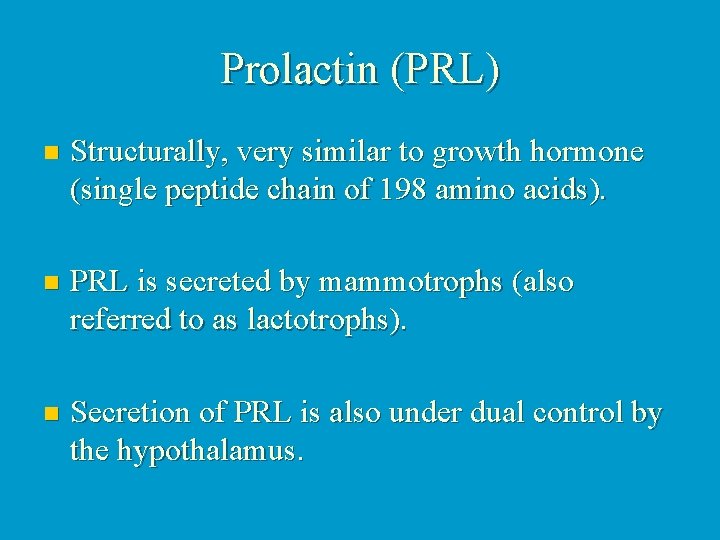
Prolactin (PRL) n Structurally, very similar to growth hormone (single peptide chain of 198 amino acids). n PRL is secreted by mammotrophs (also referred to as lactotrophs). n Secretion of PRL is also under dual control by the hypothalamus.

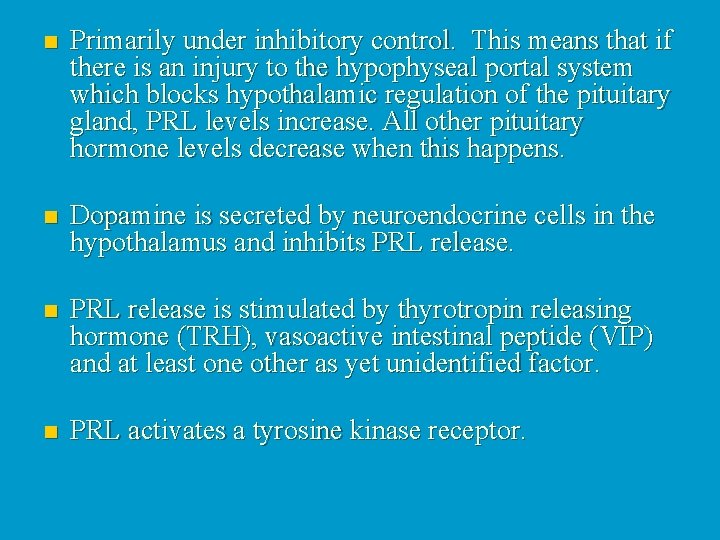
n Primarily under inhibitory control. This means that if there is an injury to the hypophyseal portal system which blocks hypothalamic regulation of the pituitary gland, PRL levels increase. All other pituitary hormone levels decrease when this happens. n Dopamine is secreted by neuroendocrine cells in the hypothalamus and inhibits PRL release. n PRL release is stimulated by thyrotropin releasing hormone (TRH), vasoactive intestinal peptide (VIP) and at least one other as yet unidentified factor. n PRL activates a tyrosine kinase receptor.

Functions of PRL: n In humans, the only effects of PRL so far identified are on reproduction and nursing. n PRL is important in stimulating differentiation of breast tissue during development. n Stimulates further development of mammary glands during pregnancy.

n Stimulates milk production (lactation) after pregnancy. n PRL has a role in regulation of the female reproductive cycle. However, its precise role has not be delineated yet. Excess PRL secretion is know to block synthesis and release of gonadotropins, disrupting menstruation and causing infertility. n PRL also can regulate male fertility, but how it does so remains unclear.

Pathophysiology of PRL secretion: n Hyposecretion is never seen. However, hyperprolactinemia (excess secretion of PRL) is a fairly common disorder. Symptoms in women usually include amenorrhea (cessation of menstruation), galactorrhea (abnormal lactation) and infertility. In men, infertility and galactorrhea are the most common symptoms. n Treatment usually consists of administration of a dopaminergic agonist, such as bromocriptine.

Thyroid Stimulating hormone (TSH) TSH is a glycoprotein hormone composed of 2 peptide chains a and b. n The a subunit is called “unspecific” because it is also incorporated into two other unrelated pituitary hormones (LH and FSH). n n The b subunit contains the biologically active sites. However, it must be combined with the a subunit in order for the hormone to be active.

n TSH secretion is controlled very tightly by the hypothalamus. n TSH secretion is stimulated by Thyrotropinreleasing hormone (TRH). TRH is a tripeptide, meaning it is composed of three amino acids. n TRH secretion is stimulated by thermal and caloric signals in the brain.

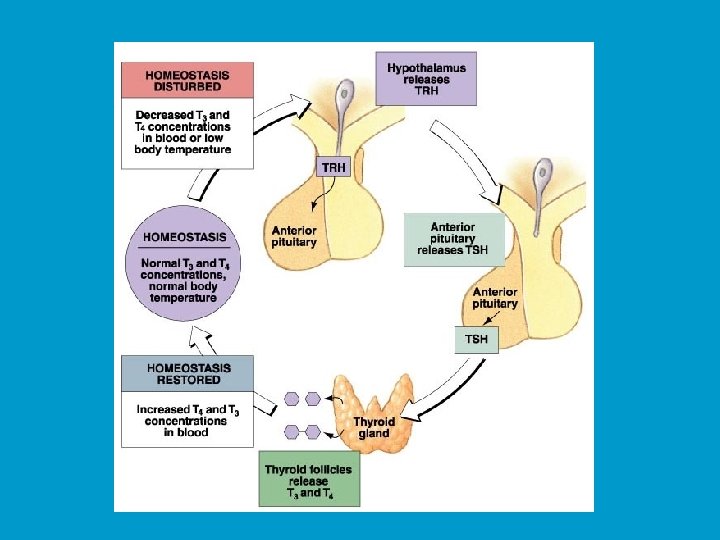
Control of TSH secretion n Negative control of TSH secretion occurs in two ways: n Triiodothyronien or T 3 (which will be discussed later) feeds back on the hypothalamus to stimulate secretion of dopamine and somatostatin. These two factors both function as TSH-release inhibiting factors. n T 3 can feed back directly onto the thyrotrophs to directly inhibit TSH secretion.

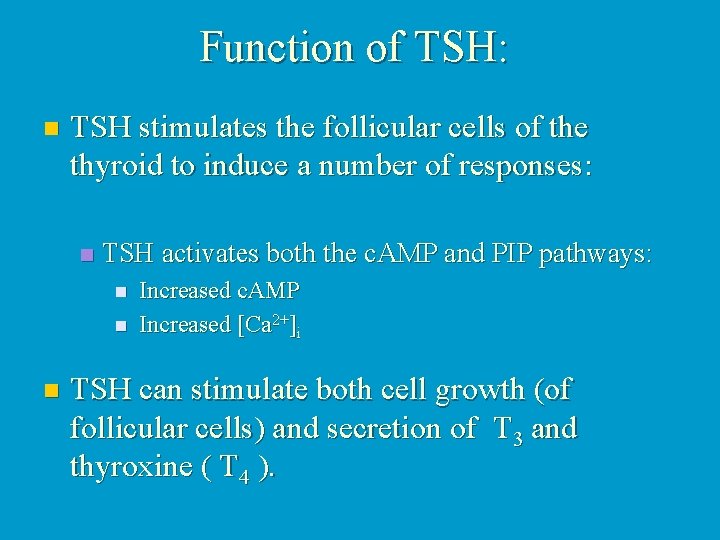
Function of TSH: n TSH stimulates the follicular cells of the thyroid to induce a number of responses: n TSH activates both the c. AMP and PIP pathways: n Increased c. AMP n Increased [Ca 2+]i n TSH can stimulate both cell growth (of follicular cells) and secretion of T 3 and thyroxine ( T 4 ).

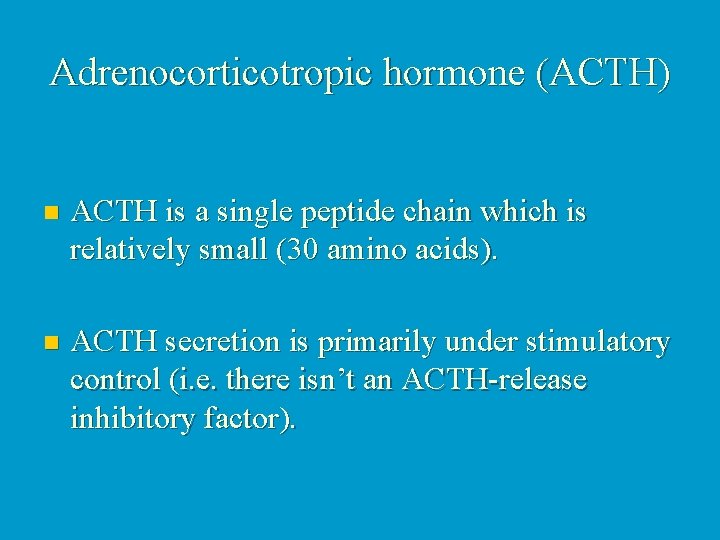
Adrenocorticotropic hormone (ACTH) n ACTH is a single peptide chain which is relatively small (30 amino acids). n ACTH secretion is primarily under stimulatory control (i. e. there isn’t an ACTH-release inhibitory factor).

n ACTH secretion is stimulated by corticotropin releasing hormone (CRH). n CRH secretion can be stimulated by a large number of factors, most of which would be considered stress factors. n Examples; infection, trauma, sleep cycle, anxiety, depression and others. (Just remember stress).

Functions of ACTH: n ACTH stimulates the adrenal gland to secrete cortisol. n ACTH levels are associated with the sleep cycle. n ACTH stimulates the c. AMP pathway in adrenocorticol cells. n ACTH can directly inhibit CRH secretion (negative feedback).

Follicular-Stimulating hormone (FSH) Luteinizing Hormone (LH) n n These are generally grouped together and called gonadotropines. Gonadotropins are secreted by the gonadotrophs, which synthesize and secrete both LH and FSH. n Both LH and FSH are peptide hormones. n Secretion of gonadotropins is mainly under positive control. n Hypothalamus secretes gonadotropin-releasing hormone (Gn. RH) which stimulates gonadotrophs to secrete both LH and FSH.

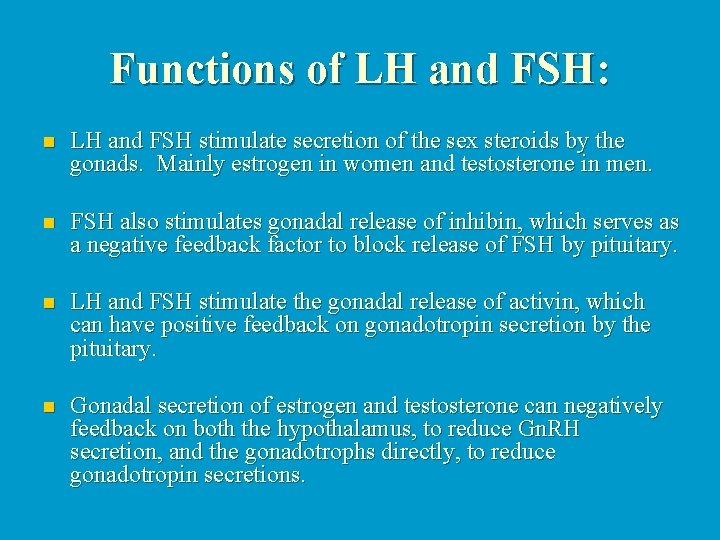
Functions of LH and FSH: n LH and FSH stimulate secretion of the sex steroids by the gonads. Mainly estrogen in women and testosterone in men. n FSH also stimulates gonadal release of inhibin, which serves as a negative feedback factor to block release of FSH by pituitary. n LH and FSH stimulate the gonadal release of activin, which can have positive feedback on gonadotropin secretion by the pituitary. n Gonadal secretion of estrogen and testosterone can negatively feedback on both the hypothalamus, to reduce Gn. RH secretion, and the gonadotrophs directly, to reduce gonadotropin secretions.

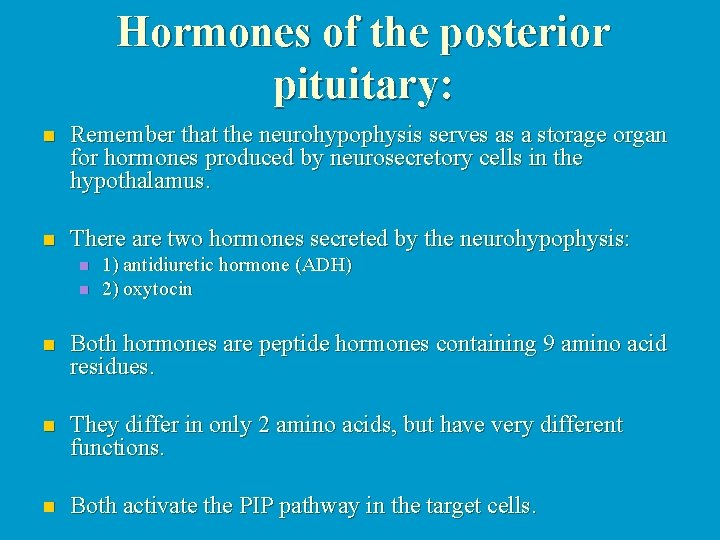
Hormones of the posterior pituitary: n Remember that the neurohypophysis serves as a storage organ for hormones produced by neurosecretory cells in the hypothalamus. n There are two hormones secreted by the neurohypophysis: n n 1) antidiuretic hormone (ADH) 2) oxytocin n Both hormones are peptide hormones containing 9 amino acid residues. n They differ in only 2 amino acids, but have very different functions. n Both activate the PIP pathway in the target cells.

ADH n Term: diuresis ö means production of urine. n ADH inhibits urine production, i. e. conserves water in the body. n Main target for ADH are the cells in the kidney which reabsorb water (will be covered in detail in the section on renal physiology). n ADH secretion is stimulated by either an increase in the osmotic concentration of the blood, or by a decrease in blood volume n usually sensed by a decrease in blood pressure.

n n n Secretion of ADH causes retention of water, which will tend to counteract both an increase in blood concentration and/or decrease in blood volume. cannot overcome serious blood loss. Conversely, excess consumption of water will have two effects: n n n increase blood volume (and pressure). decrease blood concentration. Under these conditions ADH secretion is inhibited. n n This results in formation of more urine, which is usually fairly dilute. Blood loses water and thus volume.

Oxytocin n Release of oxytocin is under neural control (like with ADH). n However, unlike ADH, the release of oxytocin is largely controlled by emotional state. n Oxytocin specifically stimulates certain smooth muscles to contract. n Primarily those of the reproductive tract and mammary glands.

n Oxytocin is required for nursing. n Principally know as the “milk letdown factor”. n It is secreted within seconds of the onset of suckling. n Sensory receptors in the nipples generate afferent impulses that stimulate the hypothalamus, triggering oxytocin secretion. n Can actually be secreted in response to auditory input, i. e. in nursing mothers in response to hearing their babies cry.

Effects of Oxytocin n Oxytocin stimulation at low doses causes rhythmic contractions of the uterus. n Oxytocin stimulation at high dose causes sustained tetanic uterine contractions. n Oxytocin is often used to induce labour.

n It is now generally believed that oxytocin produced by the fetus plays a critical role in labour. n Oxytocin is also used to stop post-partum bleeding. n The number of oxytocin receptors in uterine smooth muscles increases towards the end of pregnancy. n Oxytocin affects smooth muscle cells in uterus and vagina of non-pregnant women.

n There is clear evidence that oxytocin is involved in sexual arousal and orgasm in both men and women. n n What role it plays in men is unknown. However, it may play a strong role in reinforcing the pair-bond. The role in women is only slightly better known. n n Oxytocin is secreted in response to vaginal distention during intercourse. Oxytocin is also secreted in response to stimulation of the nipples.

Emotional considerations n Oxytocin secretion during sexual intercourse probably serves to reinforce the male-female pair-bond. n Often referred to as the “the cuddle hormone” or “the love hormone” in the popular press.

n Secretion of oxytocin during and after labour may play an important role in the formation of the mother-child pair-bond. n Oxytocin secreted during suckling may serve to reinforce this pair-bond.

n Recent studies with knock out mice has shown that oxytocin is critical in initiating and maintaining maternal care. n Oxytocin secreted in response to suckling can cause uterine contractions which may play a role in the recovery of uterine muscle tone after pregnancy and may serve to shrink the uterus back to normal.

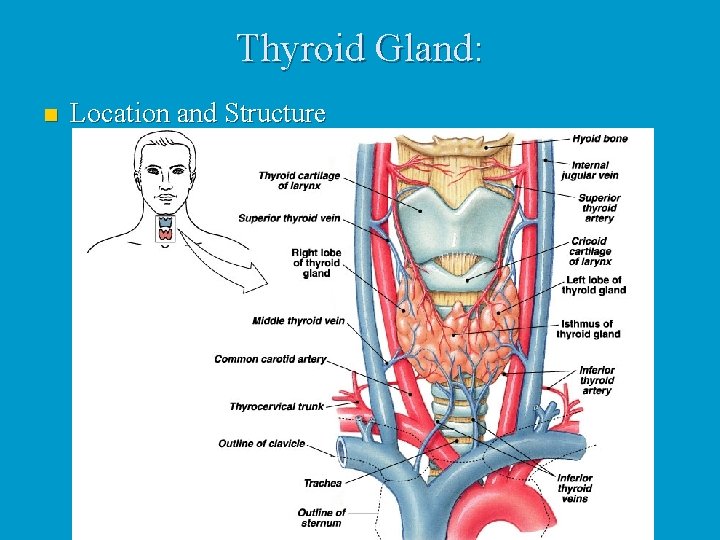
Thyroid Gland: n Location and Structure

n The largest pure endocrine gland in the body, located in the front of the neck, on the trachea just below to the larynx. n Its two lobes are connected by a median tissue mass called the isthmus. n Internally, it is composed of about 1 million of round follicles. The walls of each follice are formed by cuboidal and squamous epithelial cells called follicle cells, which produce thyroglobulin (glycoprotein).

n The lumen of each follicle stores colloid, which consists primarily of molecules of thyroglobulin. n The follicular epithelium also consists of parafollicular cells, a separate population of endocrine cells that produce calcitonin, a hormone involved in calcium homeostasis.


Thyroid hormones (THs) n The two THs contain iodine and are called thyroxin or T 4 and triiodothyronine or T 3. n T 4 and T 3 have a very similar structure as each is made up of two tyrosine amino acids linked together and either 4 or 3 atoms of iodine, respectively. n T 4 is the main hormone produced by the thyroid and T 3 has most if not all of biological activity as all target tissues rapidly convert T 4 to T 3.

n Except for the adult brain, spleen, testes, and the thyroid gland itself, THs affect all other types of cells in the body where they stimulate activity of enzymes especially those involved in glucose metabolism n Increase metabolic rate in target tissues, which increases body heat production (calorigenic effect). n THs also are critically important for normal growth and development of skeletal and nervous systems and maturation of reproductive system.

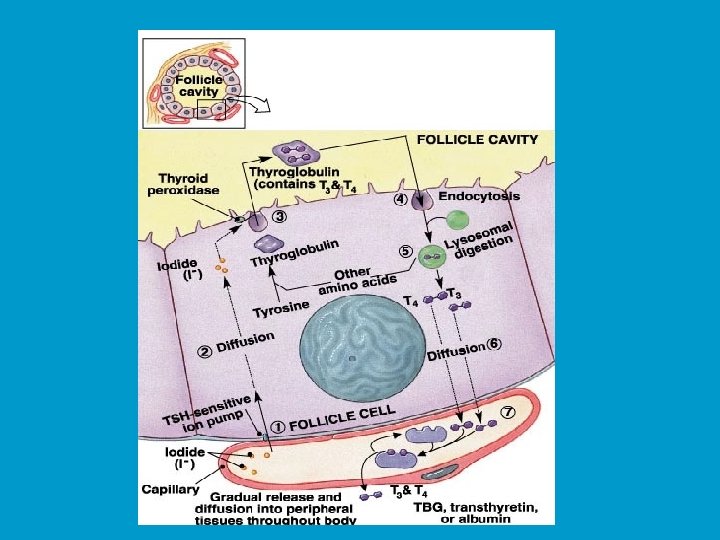
Synthesis of thyroid hormones: n Formation and storage of thyroglobulin. n This process takes place in follicle cells and the final product is packed into vesicles, their contents are discharged into the lumen of the follicle and become a major part of the colloid.

n Iodide trapping and oxidation to iodine. n To produce functional iodinated hormones, follicle cells accumulate iodide from the blood. A protein pump (iodide trap), located on the basal surface of follicle cells, actively transports iodide into follicle cells where it is oxidized and converted to iodine (I 2).

n Iodination. n Once formed, iodine is attached to tyrosine amino acids which are part of the thyroglobulin. n Iodination of one tyrosine produces monoiodotyrosine (MIT), iodination of two tyrosines diiodotyrosine (DIT).

n Coupling. n Then enzymes within the colloid link MITs and DITs in a highly specific fashion, as a result two DITs linked together result in T 4 , while coupling of MIT and DIT produce T 3.

n Coupling (cont. ) Interactions between two DITs are more frequent so more thyroxin. n At this point both thyroid hormones are still attached to thyroglobulin molecules in the colloid. n

n Colloid endocytosis. n Colloid droplets containing iodinated thyroglobulin are taken up by follicle cells by endocytosis. These combine with lysosomes to form phagolysosomes.

n Cleavage of the hormones for release. n Within the phagolysosomes, the hormones are cleaved from the thyroglobulin by lysosomal enzymes. The free hormones then diffuse through the basal membrane out of the follicle cell and into the blood stream.


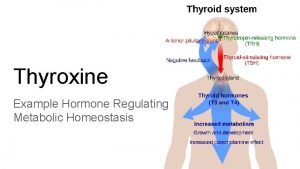
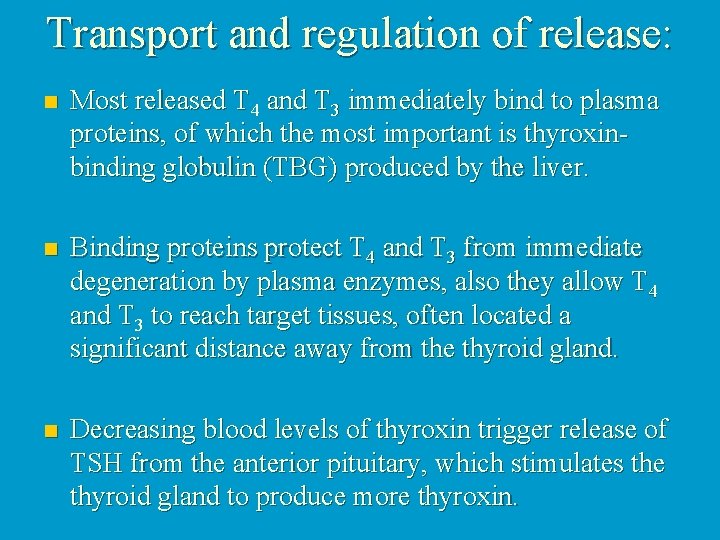
Transport and regulation of release: n Most released T 4 and T 3 immediately bind to plasma proteins, of which the most important is thyroxinbinding globulin (TBG) produced by the liver. n Binding proteins protect T 4 and T 3 from immediate degeneration by plasma enzymes, also they allow T 4 and T 3 to reach target tissues, often located a significant distance away from the thyroid gland. n Decreasing blood levels of thyroxin trigger release of TSH from the anterior pituitary, which stimulates the thyroid gland to produce more thyroxin.


Pathology of the thyroid gland function: n Both hypo- and hyperactivity and of the thyroid gland can cause severe metabolic disturbances. n In adults, hypothyroidism is referred to as n n myxedema. Symptoms: n n Low metabolic rate, poor resistance to cold temperatures, constipation, dry skin (especially facial), puffy eyes, lethargy and mental sluggishness. If hypothyroidism results from lack of iodine thyroid gland enlarges to form a goiter.

n Severe hypothyroidism during the fetal development and in infants is called cretinism. n Symptoms: A short disproportionate body, a thick tongue and neck, and mental retardation. n The condition is preventable by thyroid hormone replacement therapy. However, once developmental abnormalities and mental retardation appear, they are not reversible. n


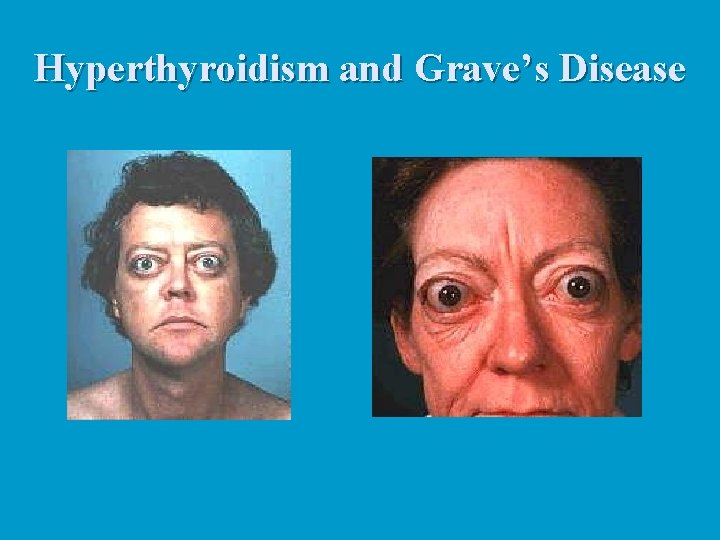
Hyperthyroidism: n The most common form of hyperthyroidism is Grave's disease, believed to be an autoimmune disease. n The immune system produces antibodies that mimic TSH, which bind to TSH receptors and permanently switch them on, resulting in continuous release of thyroid hormones. n Typical symptoms include metabolic rate, sweating, rapid and irregular heartbeat, nervousness, and weight loss despite adequate food intake. n Often, exophthalmos, or protrusion of the eyeballs, occurs caused by the edema of tissues behind the eyes followed by fibrosis. n Treatments include surgical removal of the thyroid gland (very difficult due to an extremely rich blood supply) or ingestion of radioactive iodine (131 I), which selectively destroys the most active thyroid cells.

Hyperthyroidism and Grave’s Disease

Parathyroid Glands: n The parathyroid glands are small in size and are found on the posterior aspect of the thyroid gland. n Typically, there are four of them but the actual number may vary.

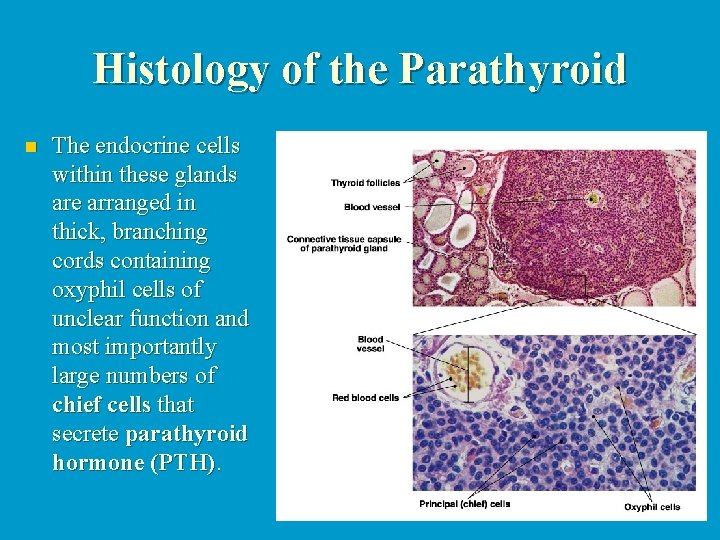
Histology of the Parathyroid n The endocrine cells within these glands are arranged in thick, branching cords containing oxyphil cells of unclear function and most importantly large numbers of chief cells that secrete parathyroid hormone (PTH).

PTH: n Small protein n Single most important hormone controlling calcium homeostasis. Its release is triggered by falling blood calcium levels and inhibited by hypercalcemia (high blood calcium). n There are three target organs for PTH: n n n skeleton kidneys intestine

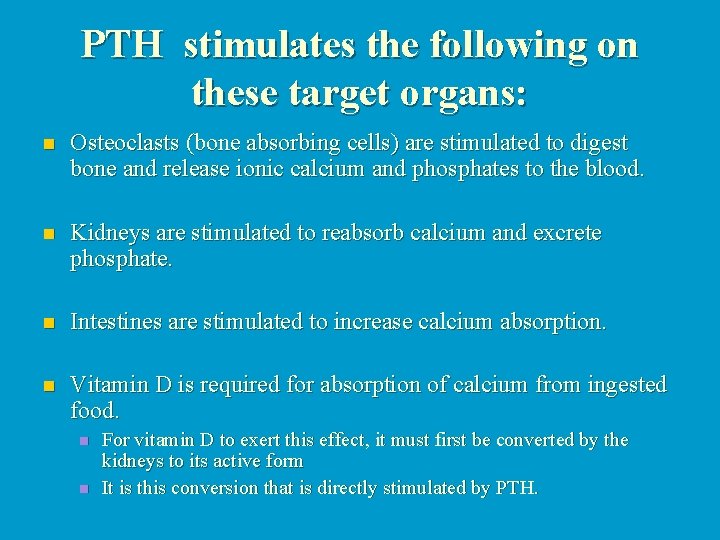
PTH stimulates the following on these target organs: n Osteoclasts (bone absorbing cells) are stimulated to digest bone and release ionic calcium and phosphates to the blood. n Kidneys are stimulated to reabsorb calcium and excrete phosphate. n Intestines are stimulated to increase calcium absorption. n Vitamin D is required for absorption of calcium from ingested food. n n For vitamin D to exert this effect, it must first be converted by the kidneys to its active form It is this conversion that is directly stimulated by PTH.



Pathology of the parathyroid glands: n Because calcium is essential for so many functions, including transmission of action potentials, muscle contraction, pacemaker activity in the heart, and blood clotting, precise control of ionic calcium levels in body fluids is absolutely critical. As a result both hyper- and hypoparathyroidism can have severe consequences.

Hyperparathyroidism: n Rare, usually the result of a parathyroid gland tumor. n Results in severe loss of calcium from the bones. n The bones soften and deform as their mineral salts are replaced by fibrous connective tissue. n Results in hypercalcemia n Leads to, depression of the nervous system leading to abnormal reflexes and weakness of the skeletal muscles, and formation of kidney stones as excess calcium salts are deposited in kidney tubules.

Hypoparathyroidism: n It is a PTH deficiency, which is a common consequence of parathyroid trauma or removal during thyroid surgery. n The resulting hypocalcemia increases excitability of neurons and may lead to tetany resulting in uncontrollable muscle twitches and convulsions, which if untreated may progress to spasms of the larynx, respiratory paralysis and death.

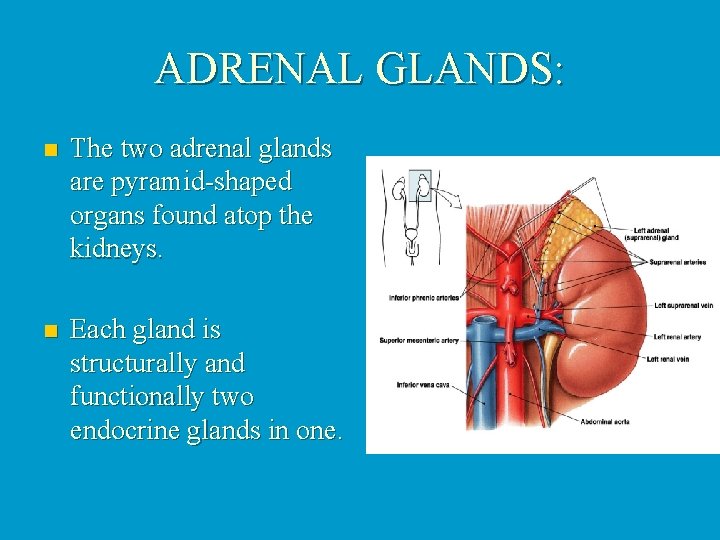
ADRENAL GLANDS: n The two adrenal glands are pyramid-shaped organs found atop the kidneys. n Each gland is structurally and functionally two endocrine glands in one.

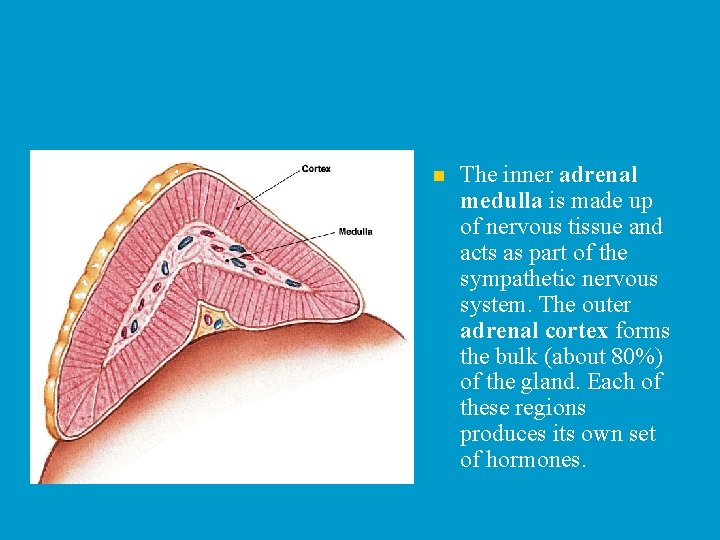
n The inner adrenal medulla is made up of nervous tissue and acts as part of the sympathetic nervous system. The outer adrenal cortex forms the bulk (about 80%) of the gland. Each of these regions produces its own set of hormones.


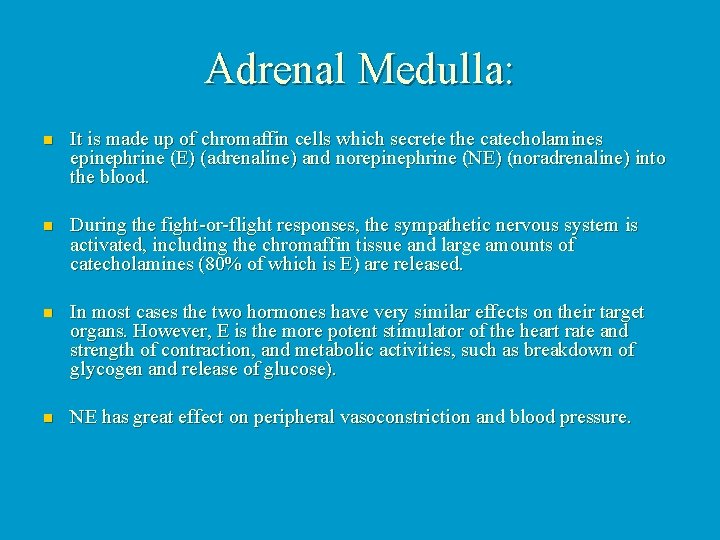
Adrenal Medulla: n It is made up of chromaffin cells which secrete the catecholamines epinephrine (E) (adrenaline) and norepinephrine (NE) (noradrenaline) into the blood. n During the fight-or-flight responses, the sympathetic nervous system is activated, including the chromaffin tissue and large amounts of catecholamines (80% of which is E) are released. n In most cases the two hormones have very similar effects on their target organs. However, E is the more potent stimulator of the heart rate and strength of contraction, and metabolic activities, such as breakdown of glycogen and release of glucose). n NE has great effect on peripheral vasoconstriction and blood pressure.


Adrenal Cortex: n n The cells of the adrenal cortex are arranged in three distinct zones, each zone producing corticosteroids. The Zona glomerulosa is the outer-most layer of cells and it produces mineralocorticoids, that help control the balance of minerals and water in the blood. The zona fasciculata is composed of cells that secrete glucocorticoids. The zona reticularis produce small amounts of adrenal sex steroids.


Hormones of the Adrenal Cortex n n Mineralocorticoids Although there are several mineralocorticoids, aldosterone is by far the most potent and accounts for more than 95% of production. Its main function is to maintain sodium balance by reducing excretion of this ion from the body. n The primary target organs of aldosterone are kidney tubules where it stimulates reabsorption of sodium ions from urine back to the bloodstream. n Aldosterone also enhances sodium absorption from sweat, saliva, and gastric juice.

n Secretion of aldosterone is induced by a number of factors such as high blood levels of potassium, low blood levels of sodium, and decreasing blood volume and pressure. n The reverse conditions inhibit secretion of aldosterone. n Glucocorticoids: n Glucocorticoids influence metabolism of most body cells, help us resist stress, and are considered to be absolutely essential to life.

n The most important glucocorticoid in humans is cortisol, but small amounts of cortisone and corticosterone are also produced. n The main effect of cortisol is to promote gluconeogenesis or formation of glucose from noncarbohydrate molecules, especially fats and proteins. n Cortisol also breaks down adipose (fat) tissue, released fatty acids can be then used by many tissues as a source of energy and "saving" glucose for the brain. n Blood levels of glucocorticoids increase significantly during stress, which helps the body to negotiate the crisis. n Interestingly, chronic excess of cortisol has significant antiinflammatory and anti-immune effects and glucocorticoid drugs are often used to control symptoms of many chronic inflammatory disorders, such as rheumatoid arthritis or allergic responses.

Regulation of glucocorticoid secretion: n n It is provided by a typical negative feedback system: increased (hypothalamus) CRH negative n increased (adenohypophysis) ACTH n increased (adrenal cortex) cortisol





n Gonadocorticoids (Sex Hormones) The amount of sex steroids produced by zona reticularis is insignificant compared to the amounts secreted by the gonads. n These hormones may contribute to the onset of puberty and the appearance of axillary and pubic hair in both males and females. n In adult women adrenal androgens (male sex hormones, especially testosterone) may be, at least partially, responsible for the sex drive. n

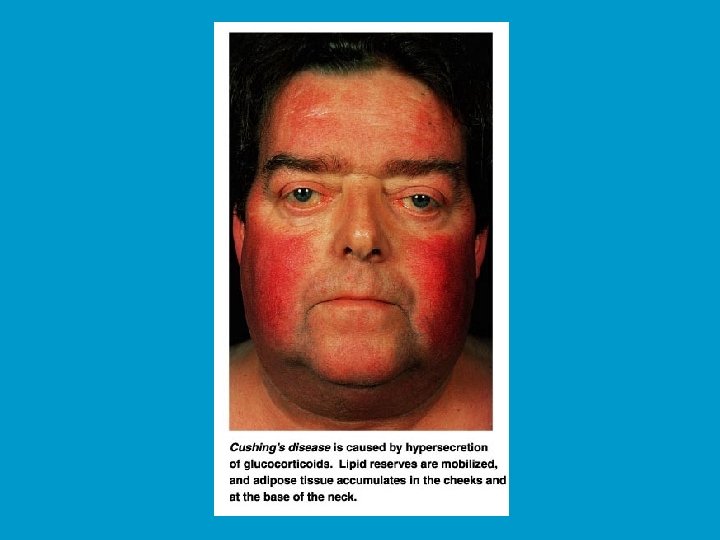
Pathology of the adrenal cortex function: Pathology of the adrenal cortex function n Hyperadrenalism : n n n It is referred to as Cushing's disease and can be caused by a cortisol-secreting tumour in the adrenal glands, ACTHsecreting tumour of the pituitary, or ACTH secreted by abdominal carcinoma. However, it most often results from the clinical administration of pharmacological (very high) doses of glucocorticoid drugs. The symptoms include a persistent hyperglycaemia, dramatic loss of muscle and bone proteins, and water and salt retention, leading to hypertension and edema - one of its signs is a swollen "moon" face. The only treatment is a surgical removal of tumour or discontinuation of the drug.


n Hypoadrenalism : n It is referred to as Addison's disease and involves significant reduction in plasma glucose and sodium, very high levels of potassium and loss of weight. The usual treatment is corticosteroid replacement therapy.


THE ENDOCRINE PANCREAS: n Located partially behind the stomach, the pancreas is a mixed gland composed of both endocrine and exocrine cells. n More than 98% of the gland is made up of acinar cells producing an enzyme-rich juice that enters a system of ducts and is delivered to the duodenum of the small intestine during food digestion.



n The remaining 1 -2% of cells form about 1 million of islets of Langerhans, tiny cell clusters that produce pancreatic hormones. n The islets have four distinct populations of cells, the two most important ones are alpha cells that produce hormone glucagon, and more numerous beta cells that synthesize insulin. In addition, delta cells produce somatostatin and F cells secrete pancreatic polypeptide (PP).

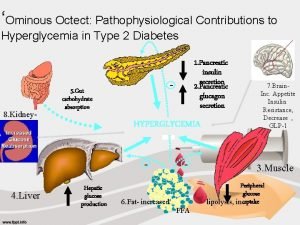
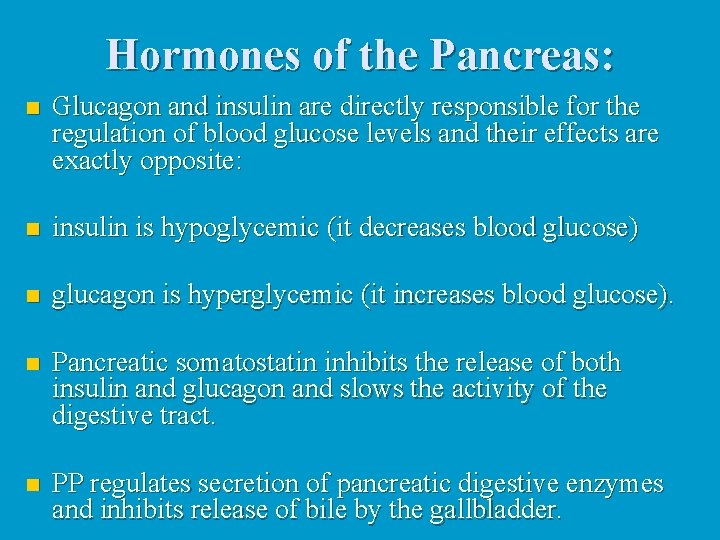
Hormones of the Pancreas: n Glucagon and insulin are directly responsible for the regulation of blood glucose levels and their effects are exactly opposite: n insulin is hypoglycemic (it decreases blood glucose) n glucagon is hyperglycemic (it increases blood glucose). n Pancreatic somatostatin inhibits the release of both insulin and glucagon and slows the activity of the digestive tract. n PP regulates secretion of pancreatic digestive enzymes and inhibits release of bile by the gallbladder.

Glucagon: n Glucagon is a 29 amino acid polypeptide with extremely potent hyperglycemic properties. One molecule of this hormone can induce the release of 100 million molecules of glucose into the blood. n The major target organ of glucagon is the liver, where it promotes: n Breakdown of glycogen to glucose (glycogenolysis) Synthesis of glucose from lactic acid and from noncarbohydrate molecules such as fatty acids and amino acids (referred to as gluconeogenesis). n Release of glucose into the blood by the liver n All these effects ↑ blood sugar levels. n Secretion of glucagon from the alpha cells is induced by, most importantly, low blood sugar levels but also by high amino acid levels in the blood (e. g. following a proteinrich meal). Rising blood sugar concentration and somatostatin from the delta cells inhibit glucagon release. n

Insulin: n Insulin is a 51 amino acid protein consisting of two polypeptide chains linked by disulfide bonds. It is synthesized as part of a larger molecule called proinsulin and packed into secretory vesicles where its middle portion is excised by enzymes to produce functional hormone, just before insulin is released from the beta cell. n As mentioned earlier, insulin's main function is to lower blood sugar levels but it also affects protein and fat metabolism. n In general, insulin: n n n Increases membrane transport of glucose into body cells, especially muscle and liver cells Inhibits the breakdown of glycogen (it should not be confused with glucagon!) into glucose, Increases the rate of ATP production from glucose Increases the rate of glycogen synthesis Increases the rate of glucose conversion to fat.

n Insulin binds to tyrosine kinase receptors, but mechanism of action, including type(s) and specific roles of second messengers, are poorly understood. n The beta cells are stimulated to produce insulin primarily by elevated blood sugar levels, but also by high blood levels of amino acids and fatty acids. n Several hormones also induce the release of insulin, including glucagon, epinephrine, growth hormone, thyroid hormones, and glucocorticoids. n In contrast, somatostatin inhibits insulin release.


 Zona fasciculata
Zona fasciculata Anterior pituitary
Anterior pituitary Endocrinology of pregnancy
Endocrinology of pregnancy Reproductive endocrinology near campbell
Reproductive endocrinology near campbell Dr betsy schwartz
Dr betsy schwartz Endocrinology
Endocrinology Definisi sistem endokrin
Definisi sistem endokrin 2232021
2232021 Reproductive biology and endocrinology
Reproductive biology and endocrinology Accenture adm made up of
Accenture adm made up of Function of antidiuretic hormone
Function of antidiuretic hormone μακροπρολακτιναιμια
μακροπρολακτιναιμια Adh role
Adh role What is a tropic hormone
What is a tropic hormone Reversal of dwarfism is caused by which hormone
Reversal of dwarfism is caused by which hormone Fasting
Fasting Metabolic action of growth hormone
Metabolic action of growth hormone Parasitism
Parasitism Luteinizing hormone in male reproductive system
Luteinizing hormone in male reproductive system Female reproductive system
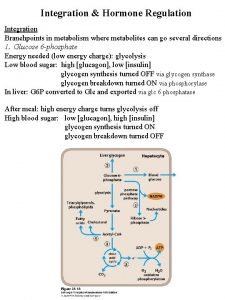
Female reproductive system Integration metabolism
Integration metabolism Parathormone function
Parathormone function Horm res paediatr
Horm res paediatr Sex hormone
Sex hormone Transcortin
Transcortin Hormone therapy
Hormone therapy Thuroid
Thuroid What is a tropic hormone
What is a tropic hormone Prolactine hormone
Prolactine hormone Kako sportsko rekreativne aktivnosti deluju na nase hormone
Kako sportsko rekreativne aktivnosti deluju na nase hormone Synthesis of insulin
Synthesis of insulin Msh
Msh Acth pathway
Acth pathway Hormone free milk walmart
Hormone free milk walmart Bromocripine
Bromocripine Thyroid hormone synthesis mnemonic
Thyroid hormone synthesis mnemonic Steroid hormones include
Steroid hormones include Hypophysis
Hypophysis Endocrine system
Endocrine system Human growth hormone effect
Human growth hormone effect Hyposecretion of growth hormone
Hyposecretion of growth hormone Hormone receptors
Hormone receptors Hormone levels during pregnancy
Hormone levels during pregnancy Peptide hormone receptors
Peptide hormone receptors Tropic hormones hypothalamus
Tropic hormones hypothalamus Neurohormones
Neurohormones Gonadotropin growth hormone
Gonadotropin growth hormone Local hormones
Local hormones Synthesis of amine hormones
Synthesis of amine hormones Calcitonin and vitamin d
Calcitonin and vitamin d The hormone adrenaline can affect only cells with *
The hormone adrenaline can affect only cells with * Lysosomees
Lysosomees Plant hormone
Plant hormone Major galactokinetic hormones
Major galactokinetic hormones Amine hormone
Amine hormone Mechanism of action of hormones
Mechanism of action of hormones Gigantism vs acromegaly
Gigantism vs acromegaly Iodine deficiency
Iodine deficiency What is plant hormone
What is plant hormone Acth
Acth Galactopoeisis
Galactopoeisis Thyroid hormone secretion
Thyroid hormone secretion Hormone
Hormone Adrenal hormone pathway
Adrenal hormone pathway Synthesis of thyroxine hormone
Synthesis of thyroxine hormone What hormone does the testes produce
What hormone does the testes produce Phosphate trashing hormone
Phosphate trashing hormone Parathyroid gland image
Parathyroid gland image Hypersecretion of growth hormone in adults
Hypersecretion of growth hormone in adults Corticotropin releasing hormone
Corticotropin releasing hormone Lipid soluble hormone
Lipid soluble hormone Patricia ferrari
Patricia ferrari Gliptins
Gliptins Anabolic rx24 composition
Anabolic rx24 composition Carbimazole side effect
Carbimazole side effect Congenital hypothyroid
Congenital hypothyroid Hormone
Hormone Hormone chart
Hormone chart Transferitin
Transferitin Thyroid dwarfism
Thyroid dwarfism Hormone lutéinisante
Hormone lutéinisante Libido
Libido Hormone secretion
Hormone secretion Types of hormone secretion
Types of hormone secretion Phycatri
Phycatri Erythropin
Erythropin Thyroid hormone receptor
Thyroid hormone receptor Sex hormone
Sex hormone Endocrine system pearson
Endocrine system pearson What is a tropic hormone
What is a tropic hormone Hormone responsible for growth
Hormone responsible for growth Plant hormone
Plant hormone Follicular carcinoma of thyroid
Follicular carcinoma of thyroid A folosit cineva female hormone blend
A folosit cineva female hormone blend Identify the hormone that is not steroid
Identify the hormone that is not steroid Intracellular hormone binding
Intracellular hormone binding Diabetic meal plan chart
Diabetic meal plan chart Rat dissection
Rat dissection Endocrine weight loss
Endocrine weight loss Q
Q Endocrine axes
Endocrine axes Parts of the endocrine system
Parts of the endocrine system Thyroid
Thyroid Endocrine
Endocrine Endocrine glands
Endocrine glands Neurohypophysis
Neurohypophysis Kliere
Kliere Pituitary and optic chiasm
Pituitary and optic chiasm Endocrine and hematologic emergencies
Endocrine and hematologic emergencies Facts about the endocrine system
Facts about the endocrine system Exocrine glands function
Exocrine glands function Chapter 16 endocrine system
Chapter 16 endocrine system Endocrine reboot chapter
Endocrine reboot chapter Hormones
Hormones Endocrine exocrine
Endocrine exocrine Lamina propria of tongue
Lamina propria of tongue Endocrine axis
Endocrine axis Endocrine parts
Endocrine parts Chapter 46 digestive and endocrine disorders
Chapter 46 digestive and endocrine disorders Endocrine system
Endocrine system Endocrine system vs nervous system
Endocrine system vs nervous system Endocrine anatomy
Endocrine anatomy Endocrine organs
Endocrine organs Thymoma cancer
Thymoma cancer Chapter 23 the endocrine system
Chapter 23 the endocrine system Whats the difference between endocrine and exocrine glands
Whats the difference between endocrine and exocrine glands Unit 6 human development lesson 1 pregnancy
Unit 6 human development lesson 1 pregnancy Endocrine system regents questions
Endocrine system regents questions Pancreas in endocrine system
Pancreas in endocrine system Are endocrine glands ductless
Are endocrine glands ductless Goitre
Goitre What are chemical signals
What are chemical signals Difference between endocrine and exocrine glands
Difference between endocrine and exocrine glands Endocrine system analogy
Endocrine system analogy Endocrine system and reproductive system
Endocrine system and reproductive system Lipid soluble hormones examples
Lipid soluble hormones examples Testosterone endocrine gland
Testosterone endocrine gland Endocrine system
Endocrine system Endocrine organ
Endocrine organ Endocrine glands
Endocrine glands Chapter 39 endocrine and reproductive systems
Chapter 39 endocrine and reproductive systems Neuroendocrine reflex
Neuroendocrine reflex Comparison of endocrine and nervous system
Comparison of endocrine and nervous system Introduction of endocrine system
Introduction of endocrine system