Endocrineactive pesticides risk to human health Hans Muilerman












- Slides: 18

Endocrine-active pesticides: risk to human health. Hans Muilerman, PAN Europe www. pan-europe. info

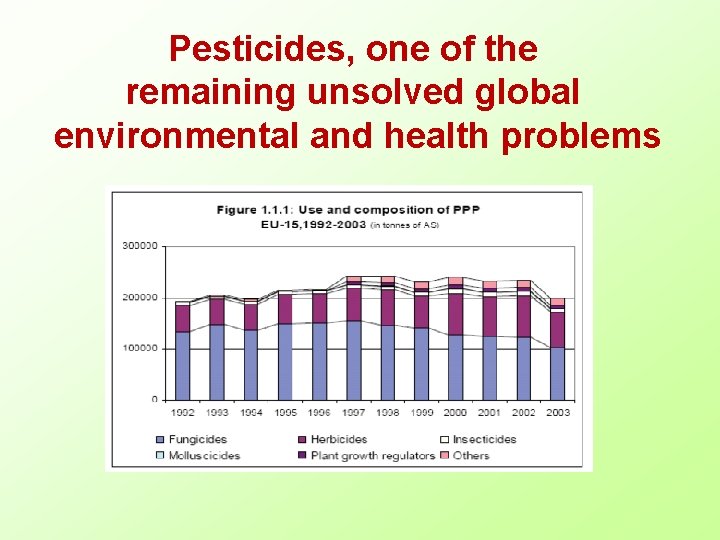
Pesticides, one of the remaining unsolved global environmental and health problems

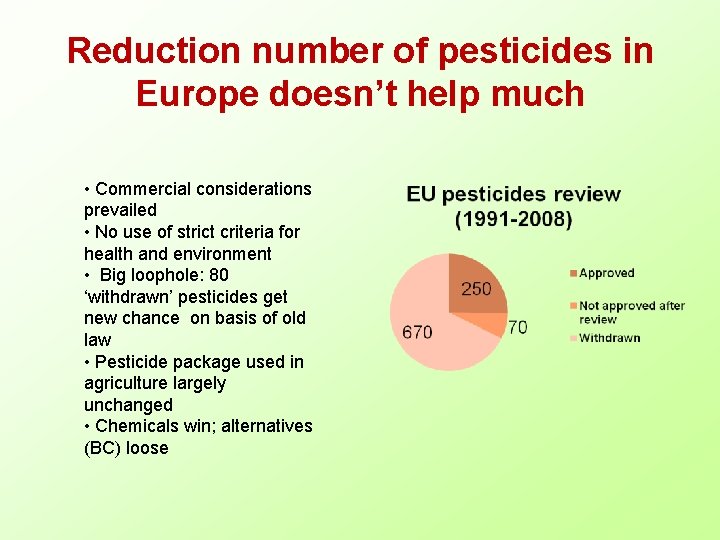
Reduction number of pesticides in Europe doesn’t help much • Commercial considerations prevailed • No use of strict criteria for health and environment • Big loophole: 80 ‘withdrawn’ pesticides get new chance on basis of old law • Pesticide package used in agriculture largely unchanged • Chemicals win; alternatives (BC) loose

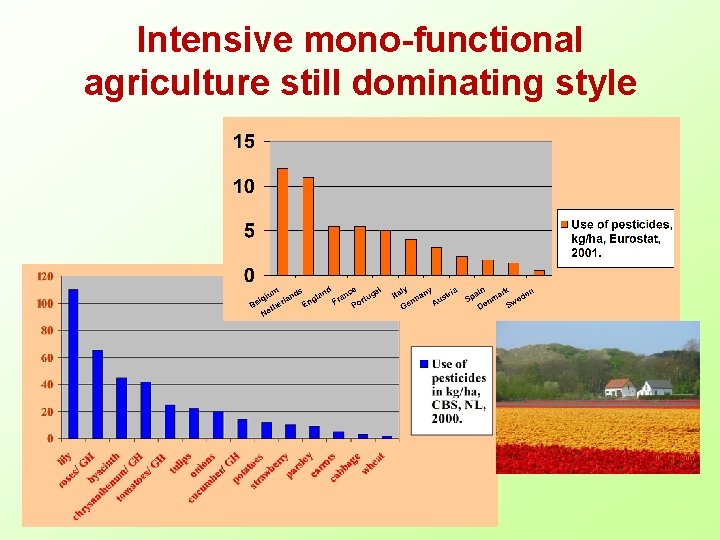
Intensive mono-functional agriculture still dominating style

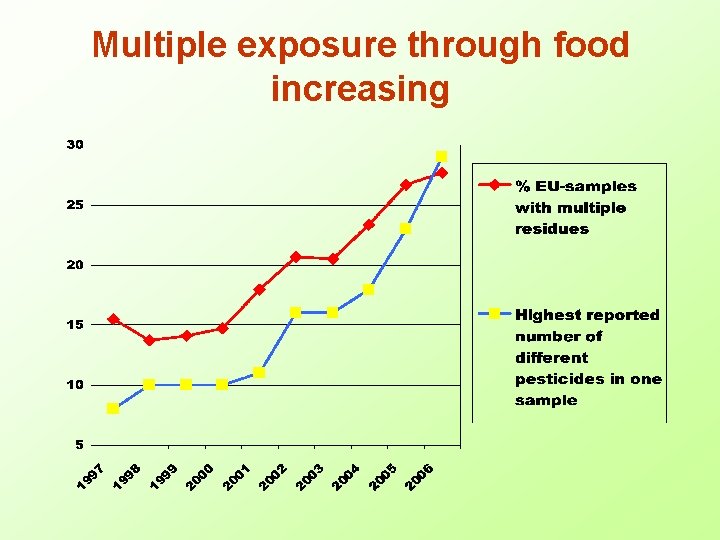
Multiple exposure through food increasing

Even actual MRL’s unsafe

EU assessment reports of pesticides (based on industry GLP) deny open literature Example Mancozeb (Thyroid eff. ): NOAEL 4, 8 mg/kg (DAR) vs. 0, 4 mg/kg, LOAEL in vivo rat, multiple tumors, (F. Belpoggi Ann. N. Y. Acad. Sci. , 2002) Example Amitrole (thyroid eff. ): NOEL 2, 9 mg/kg (DAR) vs. LOEL 0, 05 mg/l inhalation in vivo, follicular epith. hyperplasia (BKH collection) Example Picloram (anti androgen): NOAEL 20 mg/kg, US EPA (Dow) 7 mg/kg, IARC LOEL 10 mg/kg Example Linuron (anti androgen): LOAEL chosen on basis of “decreasing pituitary tumors at increasing doses” (EU DAR).

Industry-friendly climate in Brussels one reason for lack of progress. [Example from EFSA opinion 31 1 2007 on data requirements pesticides]. : • SANCO working document 2006 on data requirements “has been subject to extensive consultations with Member States and industry, whose comments have been taken into account”. • EFSA opinion arguing that a notifier should always get the opportunity to propose an alternative to a particular study, “handling of such cases will generally be assisted by dialogue between the notifier and regulatory authority at an early stage in the approval process”. • EFSA Opinion suggesting to follow ILSI HESI proposals on risk assessment (ILSI HESI is an industry dominated institute with also government people present).

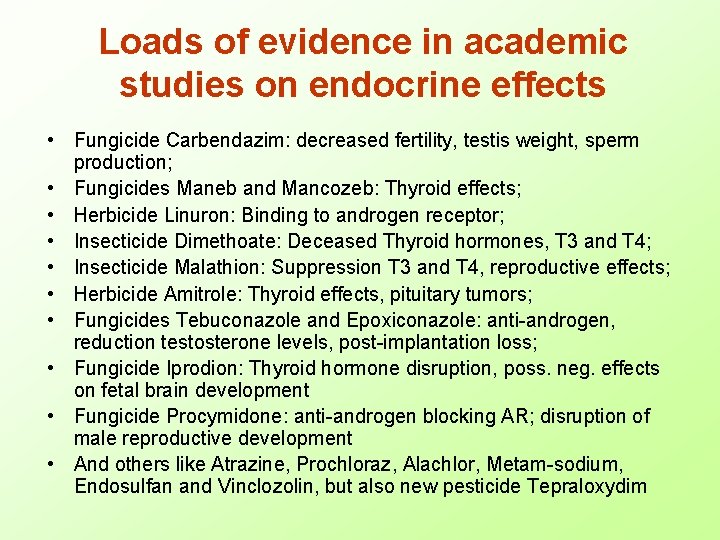
Loads of evidence in academic studies on endocrine effects • Fungicide Carbendazim: decreased fertility, testis weight, sperm production; • Fungicides Maneb and Mancozeb: Thyroid effects; • Herbicide Linuron: Binding to androgen receptor; • Insecticide Dimethoate: Deceased Thyroid hormones, T 3 and T 4; • Insecticide Malathion: Suppression T 3 and T 4, reproductive effects; • Herbicide Amitrole: Thyroid effects, pituitary tumors; • Fungicides Tebuconazole and Epoxiconazole: anti androgen, reduction testosterone levels, post implantation loss; • Fungicide Iprodion: Thyroid hormone disruption, poss. neg. effects on fetal brain development • Fungicide Procymidone: anti androgen blocking AR; disruption of male reproductive development • And others like Atrazine, Prochloraz, Alachlor, Metam sodium, Endosulfan and Vinclozolin, but also new pesticide Tepraloxydim

Potential health damage to society enormous • • • Prostate cancer (rising) Breast cancer (rising) Attention deficit hyperactivity disorder (rising) Infertility and male and female reproductive disorders, low sperm quality in parts of Europe Miscarriage Hyper allergic diseases Asthma Obesity (prevalence in infants growing) Heart disease Type 2 diabetes ………………… Are we able to prevent another asbestos disaster from happening? (early signals on asbestos 1898, ban only about 100 years later, 250. 000 – 400. 000 asbestos cancers to be expected in W-Europe in the next 35 years due to past exposures)

Old Directive was perfectly well capable of dealing with ED-pesticides Art. 4 on approval: “it is established in the light of current scientific and technological knowledge and shown from appraisal of the dossier provided for in Annex III……. it has no harmful effects on human health, directly or indirectly. Annex III, data requirements (94/79/EC), ao. the ‘musts’ • Oral 90 day study (oral 28 day study option) • Genotox in vitro (in vivo or in vitro depending outcome) • Long term toxicity and carcinogenicity (two years rat and carc. mouse) • Reproductive toxicity (2 gen rat) • Developmental toxicity studies • Delayed neurotoxicity studies (OECD 418)

So, why are endocrine effects denied so long in the EU approval system? • DG SANCO concerned getting the list done, pressed to deliver, not so much mattering how • EFSA narrow focused on DAR of Member States/ Industry • Academic studies not taken into account • Decision taking body (Standing Comm. ) voting based on agricultural, regional or commercial concerns, not always based on health concerns • Denmark and Sweden lonely heroes in trying to get EDC on the agenda (Carbendazim, Linuron). • Cumulative effects most worrying for a health point of view (recent Danish report on kids); unnecessary delay of EFSA to come up with methods

Looks like new Regulation has to realise break-through (Annex II, 3. 6. 5). • 3. 6. 5. An active substance, safener or synergist shall only be approved if, on the basis of the assessment of Community or internationally agreed test guidelines or other available data and information, including a review of the scientific literature, reviewed by the Authority, it is not considered to have endocrine disrupting properties that may cause adverse effect in humans, unless the exposure of humans to that active substance, safener or synergist in a plant protection product, under realistic proposed conditions of use, is negligible, i. e. the product is used in closed systems or in other conditions excluding contact with humans and where residues of the active substance, safener or synergist concerned on food and feed do not exceed the default value set in accordance with point (b) of Article 18(1) of Regulation (EC) No 396/2005. • Within four years from the entry into force of this Regulation, the Commission shall • • present to the Committee referred to in Article 79 (1) a draft of the measures concerning specific scientific criteria for the determination of endocrine disrupting properties to be adopted in accordance with the regulatory procedure with scrutiny referred to in Article 79(4). Pending the adoption of these criteria, substances, that are or have to be classified, in accordance with the provisions of Directive 67/548/EEC, as carcinogen category 3 and toxic for reproduction category 3, shall be considered to have endocrine disrupting properties. In addition, substances, such as those that are or have to be classified, in accordance with the provisions of Directive 67/548/EEC, as toxic for reproduction category 3 and which have toxic effects on the endocrine organs, may be considered to have such endocrine disrupting properties.

Major hurdle for effective action changing mindset on traditional RA • In testing ED chemicals we need to realize that embryo and fetus are developed under the control of hormones at parts per billion or parts per trillion. As the baby matures hormone concentrations are regulated by sensitive, thermostat like feedback control systems in the brain. Traditional RA would therefore be useless to assess ED effects to its full extend. • The endocrine system needs to be considered as an interconnected whole; all endpoints and systems (pancreas, adrenal gland, bone, mammary tissue, adipose tissue, etc. ) • Special ‘windows of vulnerability’ to be tested • Low dose effects to be taken into account. • Non monotonic dose response curves be seen as a possibility (DES and DEPH different effects at low and high dose), threshold concept needs revision • Late occurrence long after exposure has ceased assessed • Assessment of combination effects should be standard in RA, being daily reality for people

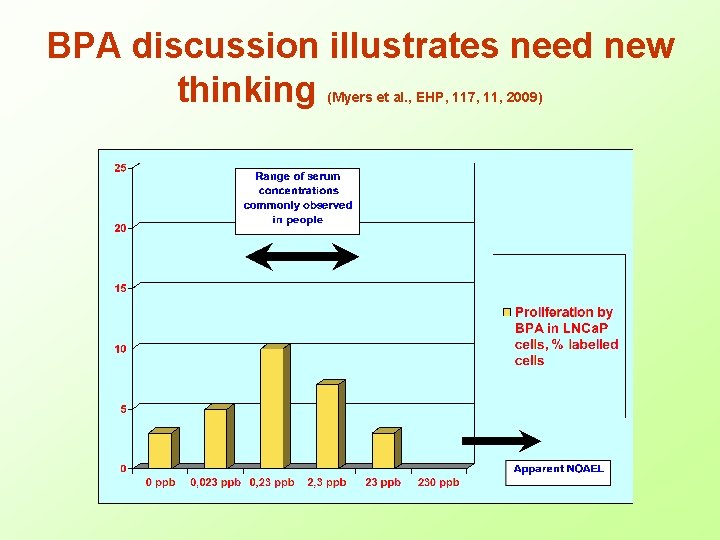
BPA discussion illustrates need new thinking (Myers et al. , EHP, 117, 11, 2009)

How to deal with industry-bias in testing? • Problems with industry GLP testing – Review of 206 studies on health of soft drinks: 0% unfavorable outcome for industry funded studies vs. 37% for no industry funding (Lesser, PLOS, 2007); – In tobacco research industry fundedstudies very 88 x more likely to conclude passive smoking is not harmful (Wise, BMJ, 1998); – Hundreds of academic studies reporting harm at low doses of Bisphenol A while all, but few, industry GLPstudies conclude BPA safe (Myers, EHP, 2009) • Academic studies vs. GLP – – – Reputation and quality of scientists in universities known Peer review of studies in international journals Studies in open literature can be replicated and discussed openly Conclusion: Academic studies should always be valued more than GLP studies, and Repeat GLP studies which differ from academic studies in independent laboratories • Involve scientists actively publishing on ED – See TEDX proposal of Theo Colborn • Asking for mechanism of action is obstruction – Even mechanism of eggshell thinning by DDT not completely elucidated – Same for imposex and molluscs – Same for smoking and long cancer

Humanity has bad track record in avoiding disasters Easter island Anasazi Maya

Let’s act now. “ If science has taught us anything, it is that the environment is full of uncertainty. It makes no sense to test it to destruction. While we wait for the doctor’s diagnosis, the patient may die” Prince Charles. And start banning the most harmful chemicals
 Hans muilerman
Hans muilerman Hans muilerman
Hans muilerman Hans muilerman
Hans muilerman Liquidity measures
Liquidity measures Cons of pesticides
Cons of pesticides Classification of insecticides
Classification of insecticides Agriscience unit 14 completion answers
Agriscience unit 14 completion answers Mediterranean diet pesticides
Mediterranean diet pesticides Different types of pesticides
Different types of pesticides Example of pesticides
Example of pesticides Bio pesticides example
Bio pesticides example Safe handling of pesticides
Safe handling of pesticides How pesticides work
How pesticides work Maine board of pesticides
Maine board of pesticides Types of pesticides
Types of pesticides Soybean aphid pesticides
Soybean aphid pesticides Integrated pest management ____ than pesticides
Integrated pest management ____ than pesticides Spider mites weed
Spider mites weed What is risk projection in software engineering
What is risk projection in software engineering































