Endocrine Treatment of Metastatic Breast Cancer New Advances

Endocrine Treatment of Metastatic Breast Cancer: New Advances; Patient Education Implications An Interactive Oncology Grand Rounds Series Joyce O’Shaughnessy, MD Chair, Breast Cancer Research Program Baylor Charles A Sammons Cancer Center Celebrating Women Chair in Breast Cancer Research Texas Oncology US Oncology Dallas, Texas
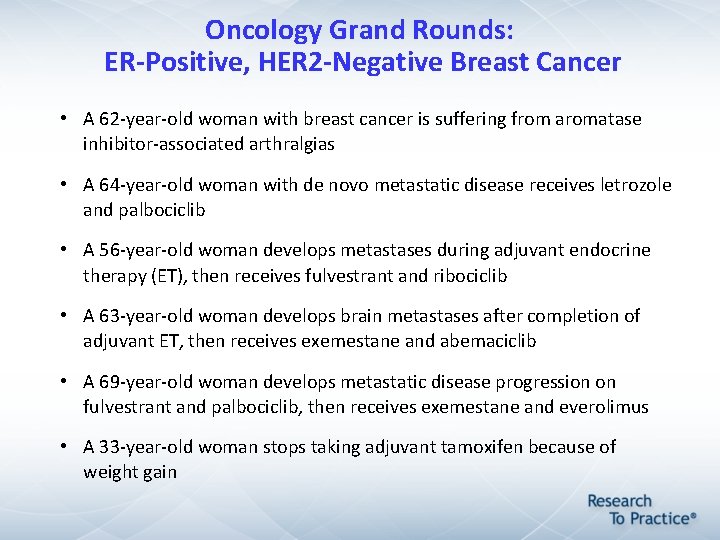
Oncology Grand Rounds: ER-Positive, HER 2 -Negative Breast Cancer • A 62 -year-old woman with breast cancer is suffering from aromatase inhibitor-associated arthralgias • A 64 -year-old woman with de novo metastatic disease receives letrozole and palbociclib • A 56 -year-old woman develops metastases during adjuvant endocrine therapy (ET), then receives fulvestrant and ribociclib • A 63 -year-old woman develops brain metastases after completion of adjuvant ET, then receives exemestane and abemaciclib • A 69 -year-old woman develops metastatic disease progression on fulvestrant and palbociclib, then receives exemestane and everolimus • A 33 -year-old woman stops taking adjuvant tamoxifen because of weight gain
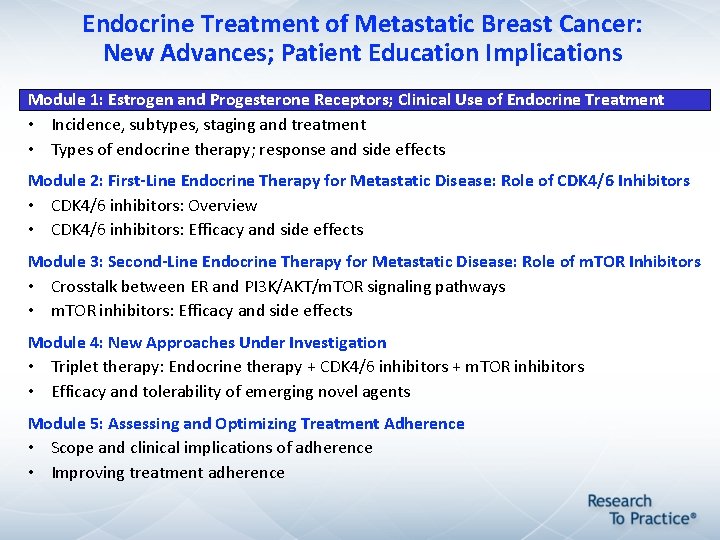
Endocrine Treatment of Metastatic Breast Cancer: New Advances; Patient Education Implications Module 1: Estrogen and Progesterone Receptors; Clinical Use of Endocrine Treatment • Incidence, subtypes, staging and treatment • Types of endocrine therapy; response and side effects Module 2: First-Line Endocrine Therapy for Metastatic Disease: Role of CDK 4/6 Inhibitors • CDK 4/6 inhibitors: Overview • CDK 4/6 inhibitors: Efficacy and side effects Module 3: Second-Line Endocrine Therapy for Metastatic Disease: Role of m. TOR Inhibitors • Crosstalk between ER and PI 3 K/AKT/m. TOR signaling pathways • m. TOR inhibitors: Efficacy and side effects Module 4: New Approaches Under Investigation • Triplet therapy: Endocrine therapy + CDK 4/6 inhibitors + m. TOR inhibitors • Efficacy and tolerability of emerging novel agents Module 5: Assessing and Optimizing Treatment Adherence • Scope and clinical implications of adherence • Improving treatment adherence
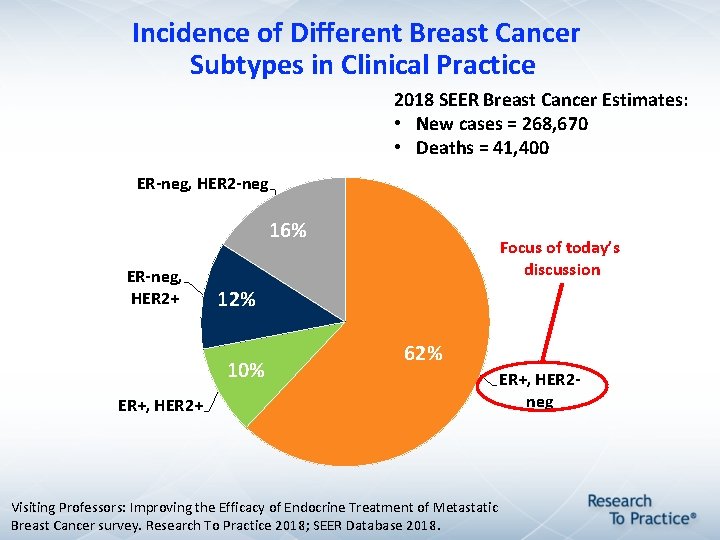
Incidence of Different Breast Cancer Subtypes in Clinical Practice 2018 SEER Breast Cancer Estimates: • New cases = 268, 670 • Deaths = 41, 400 ER-neg, HER 2 -neg 16% ER-neg, HER 2+ 12% 10% ER+, HER 2+ Focus of today’s discussion 62% ER+, HER 2 neg Visiting Professors: Improving the Efficacy of Endocrine Treatment of Metastatic Breast Cancer survey. Research To Practice 2018; SEER Database 2018.
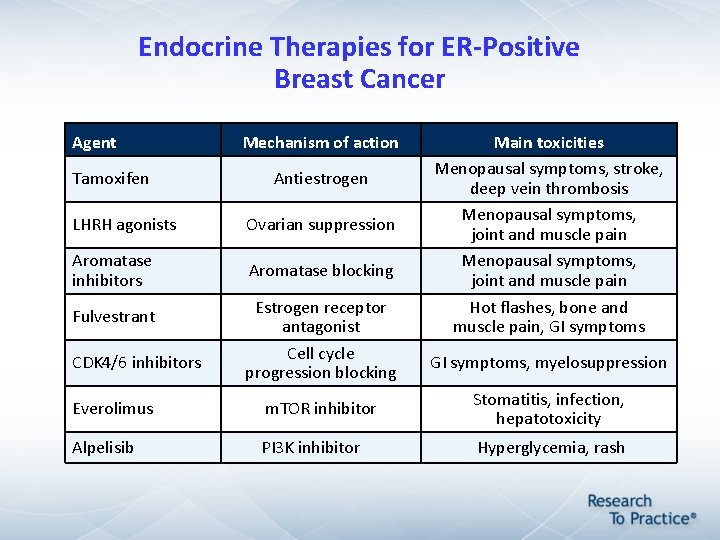
Endocrine Therapies for ER-Positive Breast Cancer Agent Tamoxifen Mechanism of action Antiestrogen LHRH agonists Ovarian suppression Aromatase inhibitors Aromatase blocking Fulvestrant CDK 4/6 inhibitors Estrogen receptor antagonist Cell cycle progression blocking Main toxicities Menopausal symptoms, stroke, deep vein thrombosis Menopausal symptoms, joint and muscle pain Hot flashes, bone and muscle pain, GI symptoms, myelosuppression Everolimus m. TOR inhibitor Stomatitis, infection, hepatotoxicity Alpelisib PI 3 K inhibitor Hyperglycemia, rash
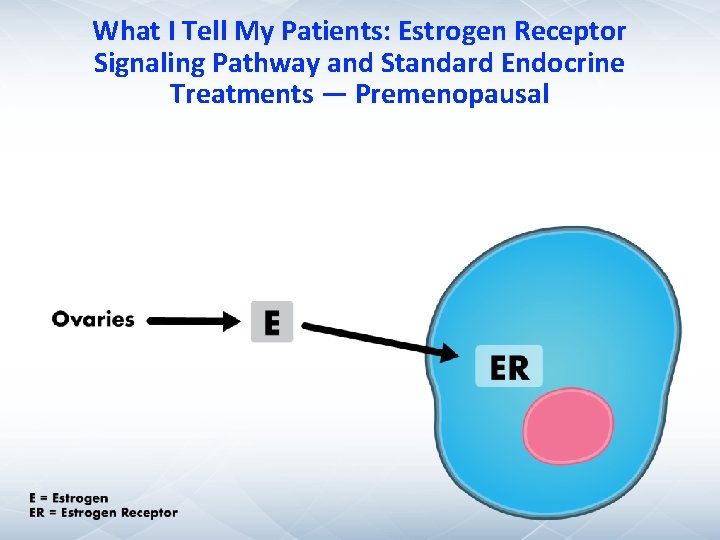
What I Tell My Patients: Estrogen Receptor Signaling Pathway and Standard Endocrine Treatments — Premenopausal
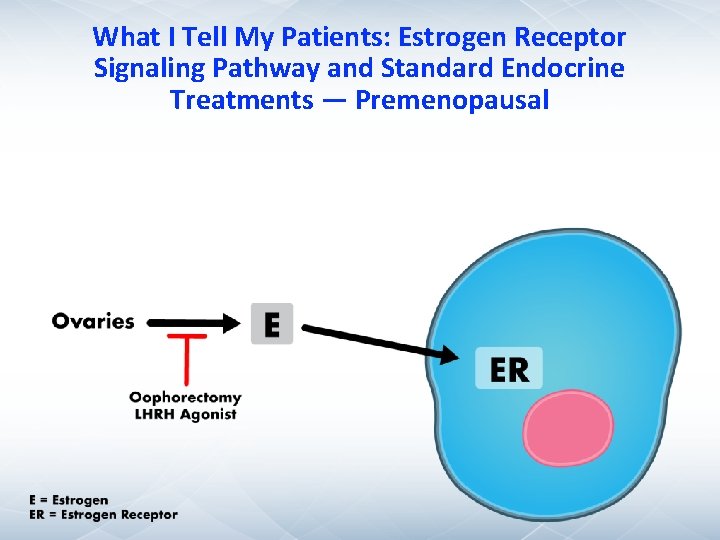
What I Tell My Patients: Estrogen Receptor Signaling Pathway and Standard Endocrine Treatments — Premenopausal
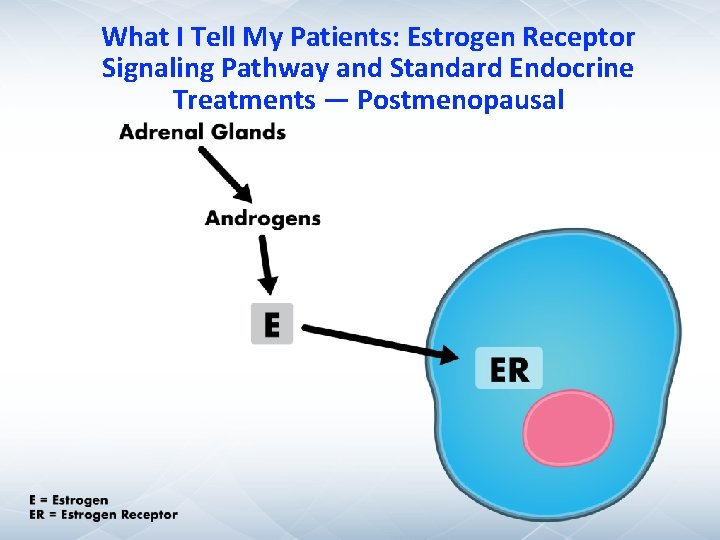
What I Tell My Patients: Estrogen Receptor Signaling Pathway and Standard Endocrine Treatments — Postmenopausal
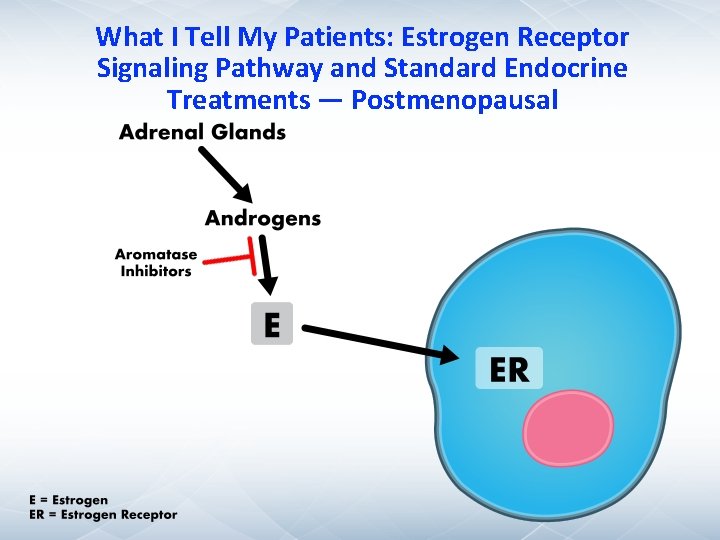
What I Tell My Patients: Estrogen Receptor Signaling Pathway and Standard Endocrine Treatments — Postmenopausal
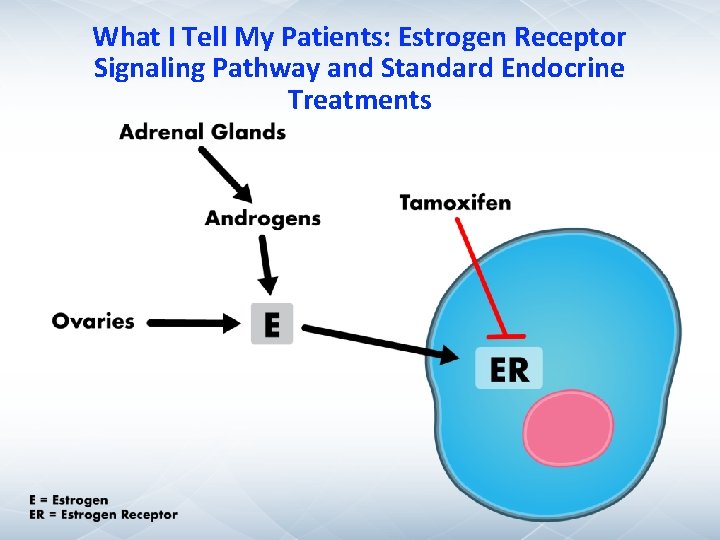
What I Tell My Patients: Estrogen Receptor Signaling Pathway and Standard Endocrine Treatments
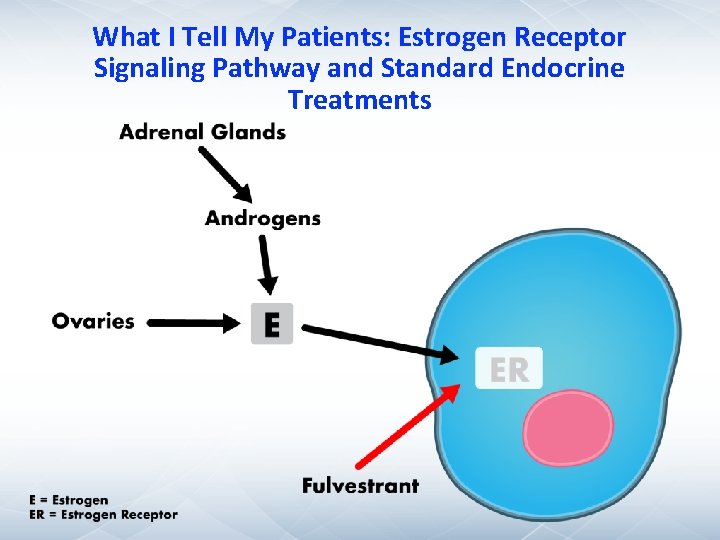
What I Tell My Patients: Estrogen Receptor Signaling Pathway and Standard Endocrine Treatments
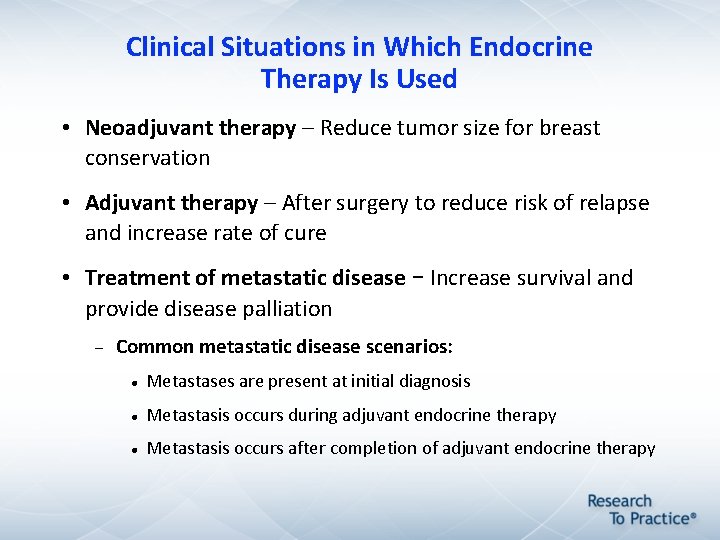
Clinical Situations in Which Endocrine Therapy Is Used • Neoadjuvant therapy – Reduce tumor size for breast conservation • Adjuvant therapy – After surgery to reduce risk of relapse and increase rate of cure • Treatment of metastatic disease – Increase survival and provide disease palliation Common metastatic disease scenarios: Metastases are present at initial diagnosis Metastasis occurs during adjuvant endocrine therapy Metastasis occurs after completion of adjuvant endocrine therapy
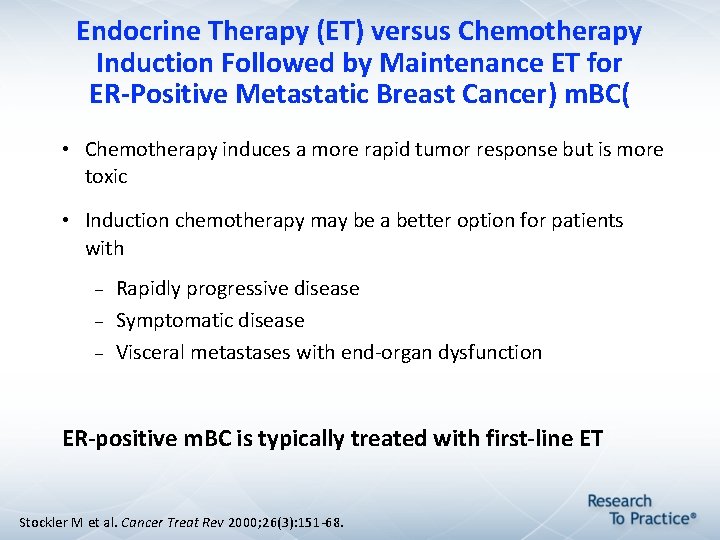
Endocrine Therapy (ET) versus Chemotherapy Induction Followed by Maintenance ET for ER-Positive Metastatic Breast Cancer) m. BC( • Chemotherapy induces a more rapid tumor response but is more toxic • Induction chemotherapy may be a better option for patients with Rapidly progressive disease Symptomatic disease Visceral metastases with end-organ dysfunction ER-positive m. BC is typically treated with first-line ET Stockler M et al. Cancer Treat Rev 2000; 26(3): 151 -68.

Case 1 • 62 yo F with 2. 8 -cm moderately differentiated cancer (ER+, PR+, HER 2 1+) s/p lumpectomy and SLNbx (0/2 LN+) Oncotype DX® 14 Received postlumpectomy XRT • 2/2016 Started on letrozole Initially tolerated well with mild hot flashes and mild morning stiffness in fingers and knees Gradually had worsening arthralgias in fingers, shoulders, knees, feet Pain limited activities Tried NSAID with only minimal relief

Case 1 • 5/2016 Held letrozole for 3 weeks, pain improved Switched to exemestane Pain recurred Developed carpal tunnel symptoms Tried duloxetine with minimal improvement • 8/2016 Stopped exemestane • 9/2016 Started tamoxifen – arthralgias still mild problem but no longer limiting
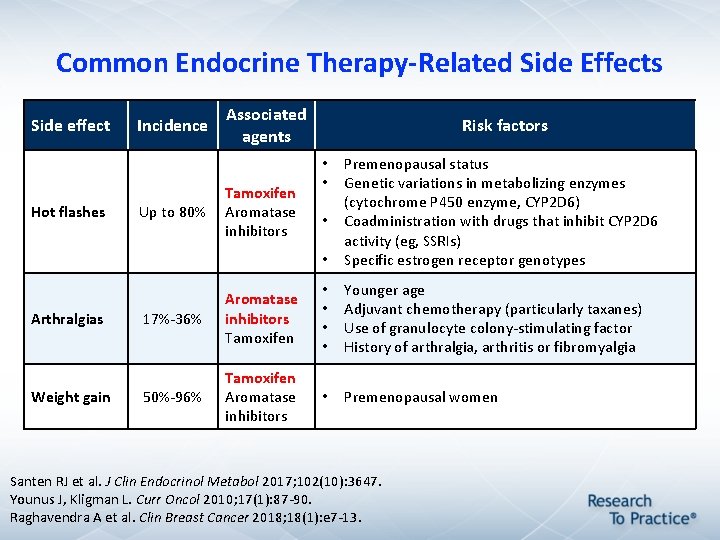
Common Endocrine Therapy-Related Side Effects Side effect Hot flashes Arthralgias Weight gain Incidence Associated agents Risk factors • Premenopausal status Genetic variations in metabolizing enzymes (cytochrome P 450 enzyme, CYP 2 D 6) Coadministration with drugs that inhibit CYP 2 D 6 activity (eg, SSRIs) Specific estrogen receptor genotypes 17%-36% Aromatase inhibitors Tamoxifen • • Younger age Adjuvant chemotherapy (particularly taxanes) Use of granulocyte colony-stimulating factor History of arthralgia, arthritis or fibromyalgia 50%-96% Tamoxifen Aromatase inhibitors • Premenopausal women Up to 80% Tamoxifen Aromatase inhibitors • • • Santen RJ et al. J Clin Endocrinol Metabol 2017; 102(10): 3647. Younus J, Kligman L. Curr Oncol 2010; 17(1): 87 -90. Raghavendra A et al. Clin Breast Cancer 2018; 18(1): e 7 -13.
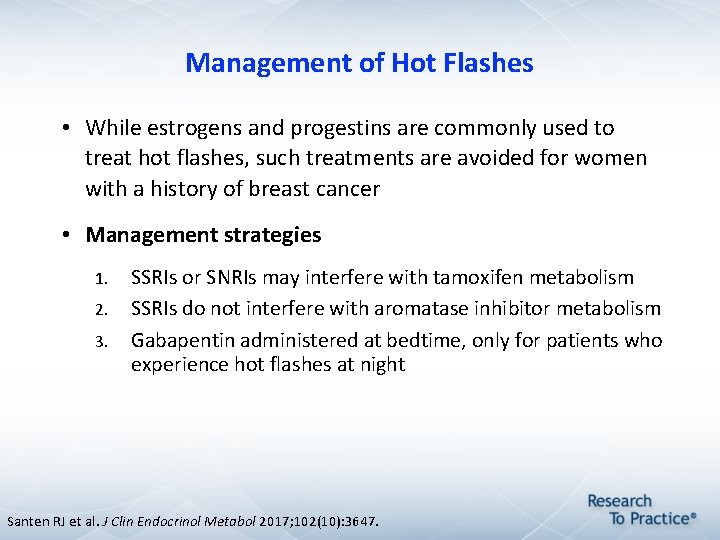
Management of Hot Flashes • While estrogens and progestins are commonly used to treat hot flashes, such treatments are avoided for women with a history of breast cancer • Management strategies 1. 2. 3. SSRIs or SNRIs may interfere with tamoxifen metabolism SSRIs do not interfere with aromatase inhibitor metabolism Gabapentin administered at bedtime, only for patients who experience hot flashes at night Santen RJ et al. J Clin Endocrinol Metabol 2017; 102(10): 3647.

Management of Aromatase Inhibitor-Induced Arthralgias • Lifestyle modifications: weight loss, exercise, morning warmups • Physical therapy/occupational therapy • Pain relievers • Complementary treatment: Vitamin D Massage therapy Glucosamine Chondroitin Zeel® injection, acupuncture, hypnosis, et cetera Younus J, Kligman L. Curr Oncol 2010; 17(1): 87 -90.
Endocrine Treatment of Metastatic Breast Cancer: New Advances; Patient Education Implications Module 1: Estrogen and Progesterone Receptors; Clinical Use of Endocrine Treatment • Incidence, subtypes, staging and treatment • Types of endocrine therapy; response and side effects Module 2: First-Line Endocrine Therapy for Metastatic Disease: Role of CDK 4/6 Inhibitors • CDK 4/6 inhibitors: Overview • CDK 4/6 inhibitors: Efficacy and side effects Module 3: Second-Line Endocrine Therapy for Metastatic Disease: Role of m. TOR Inhibitors • Crosstalk between ER and PI 3 K/AKT/m. TOR signaling pathways • m. TOR inhibitors: Efficacy and side effects Module 4: New Approaches Under Investigation • Triplet therapy: Endocrine therapy + CDK 4/6 inhibitors + m. TOR inhibitors • Efficacy and tolerability of emerging novel agents Module 5: Assessing and Optimizing Treatment Adherence • Scope and clinical implications of adherence • Improving treatment adherence

Case 2 • 64 yo F presented to local ED with RUQ pain in 1/2017. CT showed no gall bladder pathology but 2 liver lesions 2. 1 and 1. 5 cm. Bx of liver showed adeno. CA ER+, PR+, HER 2 2+, FISH-. Mammogram showed 3 -cm R UOQ lesion. Bx showed same path as above. LFTs WNL. CA 15. 3 112 • 2/17 Started on letrozole + palbociclib • 3/17 Tolerating well clinically but ANC 700 on C 2 D 1 Reduced dose from 125 mg to 100 mg • 4/17 ANC 950 – continued at 100 mg CA 15. 3 64 • 6/17 CT: liver lesions 1. 1 and 0. 5 cm, no new lesions CA 15. 3 44
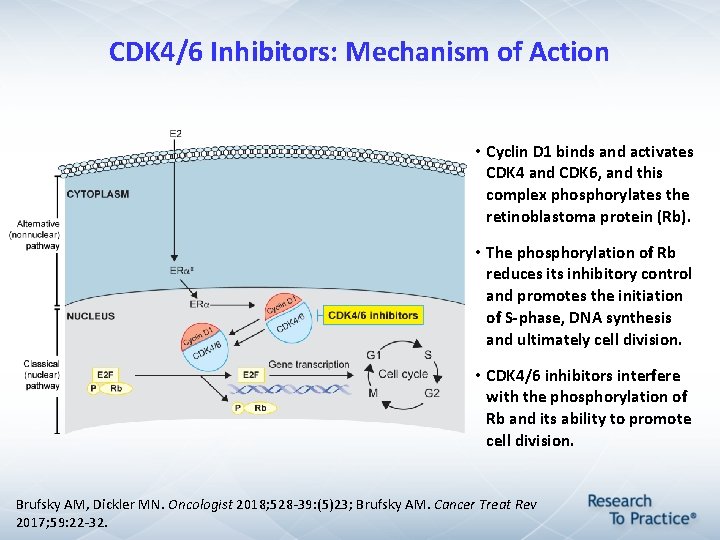
CDK 4/6 Inhibitors: Mechanism of Action • Cyclin D 1 binds and activates CDK 4 and CDK 6, and this complex phosphorylates the retinoblastoma protein (Rb). • The phosphorylation of Rb reduces its inhibitory control and promotes the initiation of S-phase, DNA synthesis and ultimately cell division. • CDK 4/6 inhibitors interfere with the phosphorylation of Rb and its ability to promote cell division. Brufsky AM, Dickler MN. Oncologist 2018; 528 -39: (5)23; Brufsky AM. Cancer Treat Rev 2017; 59: 22 -32.
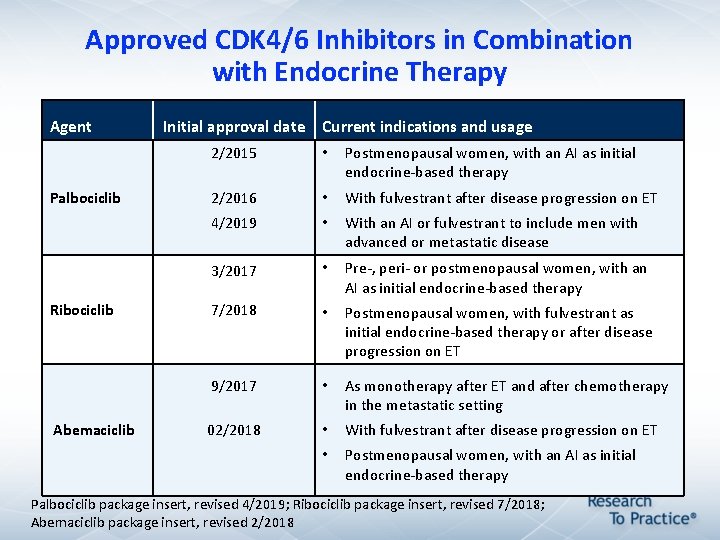
Approved CDK 4/6 Inhibitors in Combination with Endocrine Therapy Agent Palbociclib Ribociclib Abemaciclib Initial approval date Current indications and usage 2/2015 • Postmenopausal women, with an AI as initial endocrine-based therapy 2/2016 • With fulvestrant after disease progression on ET 4/2019 • With an AI or fulvestrant to include men with advanced or metastatic disease 3/2017 • Pre-, peri- or postmenopausal women, with an AI as initial endocrine-based therapy 7/2018 • Postmenopausal women, with fulvestrant as initial endocrine-based therapy or after disease progression on ET 9/2017 • As monotherapy after ET and after chemotherapy in the metastatic setting 02/2018 • With fulvestrant after disease progression on ET • Postmenopausal women, with an AI as initial endocrine-based therapy Palbociclib package insert, revised 4/2019; Ribociclib package insert, revised 7/2018; Abemaciclib package insert, revised 2/2018
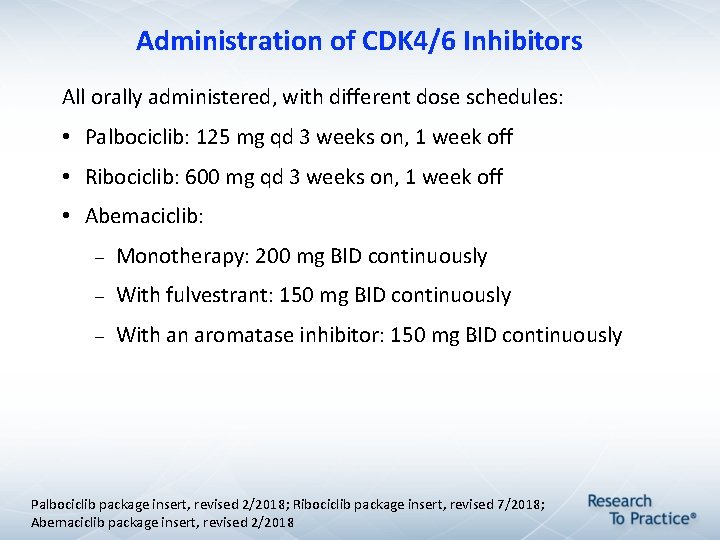
Administration of CDK 4/6 Inhibitors All orally administered, with different dose schedules: • Palbociclib: 125 mg qd 3 weeks on, 1 week off • Ribociclib: 600 mg qd 3 weeks on, 1 week off • Abemaciclib: Monotherapy: 200 mg BID continuously With fulvestrant: 150 mg BID continuously With an aromatase inhibitor: 150 mg BID continuously Palbociclib package insert, revised 2/2018; Ribociclib package insert, revised 7/2018; Abemaciclib package insert, revised 2/2018
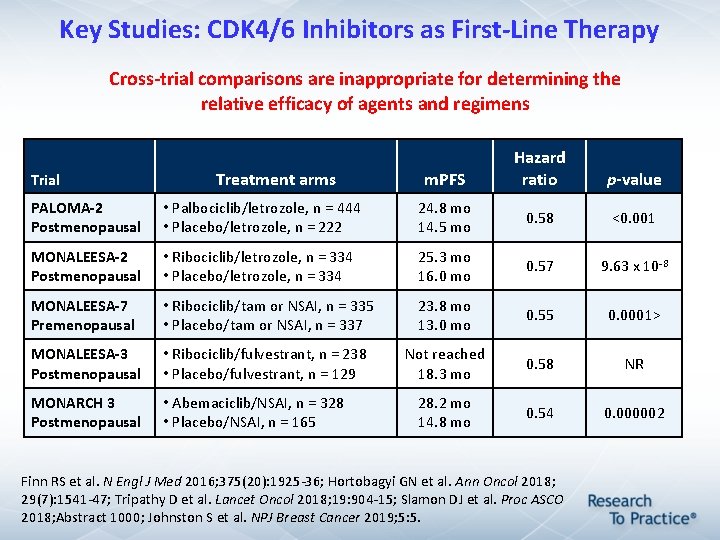
Key Studies: CDK 4/6 Inhibitors as First-Line Therapy Cross-trial comparisons are inappropriate for determining the relative efficacy of agents and regimens Trial Treatment arms m. PFS Hazard ratio p-value PALOMA-2 Postmenopausal • Palbociclib/letrozole, n = 444 • Placebo/letrozole, n = 222 24. 8 mo 14. 5 mo 0. 58 <0. 001 MONALEESA-2 Postmenopausal • Ribociclib/letrozole, n = 334 • Placebo/letrozole, n = 334 25. 3 mo 16. 0 mo 0. 57 9. 63 x 10 -8 MONALEESA-7 Premenopausal • Ribociclib/tam or NSAI, n = 335 • Placebo/tam or NSAI, n = 337 23. 8 mo 13. 0 mo 0. 55 0. 0001> MONALEESA-3 Postmenopausal • Ribociclib/fulvestrant, n = 238 • Placebo/fulvestrant, n = 129 Not reached 18. 3 mo 0. 58 NR MONARCH 3 Postmenopausal • Abemaciclib/NSAI, n = 328 • Placebo/NSAI, n = 165 28. 2 mo 14. 8 mo 0. 54 0. 000002 Finn RS et al. N Engl J Med 2016; 375(20): 1925 -36; Hortobagyi GN et al. Ann Oncol 2018; 29(7): 1541 -47; Tripathy D et al. Lancet Oncol 2018; 19: 904 -15; Slamon DJ et al. Proc ASCO 2018; Abstract 1000; Johnston S et al. NPJ Breast Cancer 2019; 5: 5.
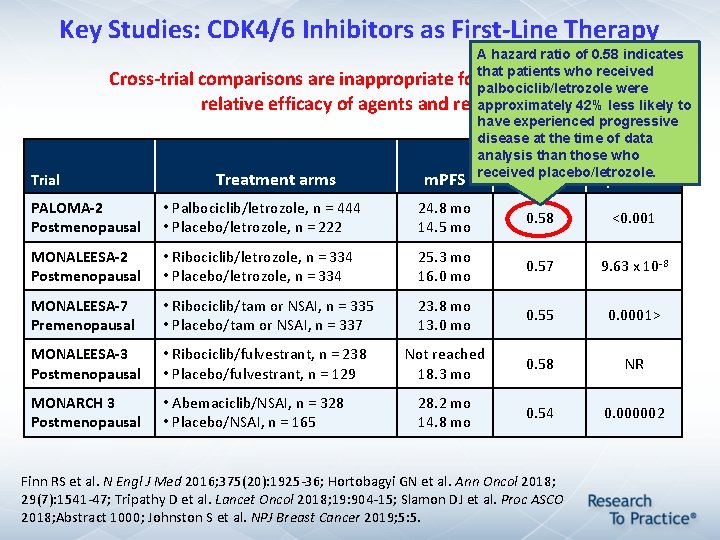
Key Studies: CDK 4/6 Inhibitors as First-Line Therapy Trial A hazard ratio of 0. 58 indicates that patients who received Cross-trial comparisons are inappropriate for determining the palbociclib/letrozole were approximately 42% less likely to relative efficacy of agents and regimens have experienced progressive disease at the time of data analysis than those who Hazard received placebo/letrozole. Treatment arms m. PFS ratio p-value PALOMA-2 Postmenopausal • Palbociclib/letrozole, n = 444 • Placebo/letrozole, n = 222 24. 8 mo 14. 5 mo 0. 58 <0. 001 MONALEESA-2 Postmenopausal • Ribociclib/letrozole, n = 334 • Placebo/letrozole, n = 334 25. 3 mo 16. 0 mo 0. 57 9. 63 x 10 -8 MONALEESA-7 Premenopausal • Ribociclib/tam or NSAI, n = 335 • Placebo/tam or NSAI, n = 337 23. 8 mo 13. 0 mo 0. 55 0. 0001> MONALEESA-3 Postmenopausal • Ribociclib/fulvestrant, n = 238 • Placebo/fulvestrant, n = 129 Not reached 18. 3 mo 0. 58 NR MONARCH 3 Postmenopausal • Abemaciclib/NSAI, n = 328 • Placebo/NSAI, n = 165 28. 2 mo 14. 8 mo 0. 54 0. 000002 Finn RS et al. N Engl J Med 2016; 375(20): 1925 -36; Hortobagyi GN et al. Ann Oncol 2018; 29(7): 1541 -47; Tripathy D et al. Lancet Oncol 2018; 19: 904 -15; Slamon DJ et al. Proc ASCO 2018; Abstract 1000; Johnston S et al. NPJ Breast Cancer 2019; 5: 5.
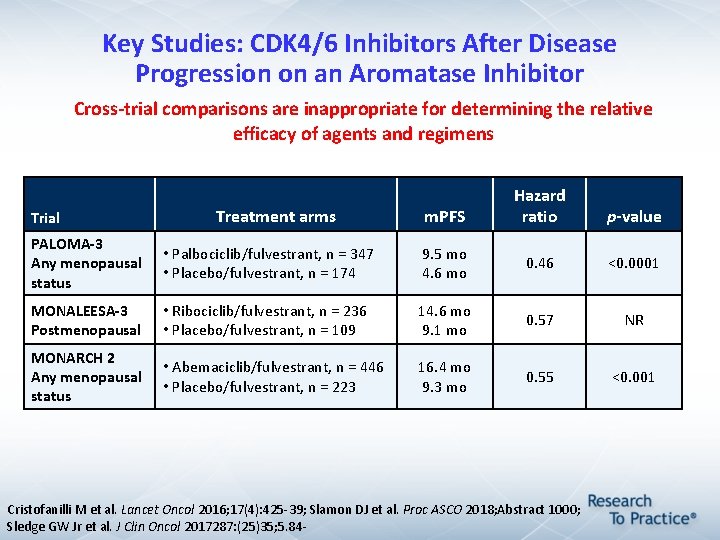
Key Studies: CDK 4/6 Inhibitors After Disease Progression on an Aromatase Inhibitor Cross-trial comparisons are inappropriate for determining the relative efficacy of agents and regimens Trial Treatment arms m. PFS Hazard ratio p-value PALOMA-3 Any menopausal status • Palbociclib/fulvestrant, n = 347 • Placebo/fulvestrant, n = 174 9. 5 mo 4. 6 mo 0. 46 <0. 0001 MONALEESA-3 Postmenopausal • Ribociclib/fulvestrant, n = 236 • Placebo/fulvestrant, n = 109 14. 6 mo 9. 1 mo 0. 57 NR MONARCH 2 Any menopausal status • Abemaciclib/fulvestrant, n = 446 • Placebo/fulvestrant, n = 223 16. 4 mo 9. 3 mo 0. 55 <0. 001 Cristofanilli M et al. Lancet Oncol 2016; 17(4): 425 -39; Slamon DJ et al. Proc ASCO 2018; Abstract 1000; Sledge GW Jr et al. J Clin Oncol 2017287: (25)35; 5. 84 -

CDK 4/6 Inhibitors: Select ASCO 2019 Presentations • Hurvitz S et al. Phase III MONALEESA-7 trial of premenopausal patients with HR+/HER 2− advanced breast cancer (ABC) treated with endocrine therapy ± ribociclib: Overall survival (OS) results. Abstract LBA 1008. • O’Leary B et al. Genomic markers of early progression on fulvestrant with or without palbociclib for ER+ advanced breast cancer in the PALOMA-3 trial. Abstract 1010. • Razavi P et al. Molecular profiling of ER+ metastatic breast cancers to reveal association of genomic alterations with acquired resistance to CDK 4/6 inhibitors. Abstract 1009. • Park Y et al. A randomized phase II study of palbociclib plus exemestane with GNRH agonist versus capecitabine in premenopausal women with hormone receptor-positive metastatic breast cancer (KCSG-BR 15 -10, NCT 02592746). Abstract 1007.

Case 3 • 6/13 56 yo F with 3. 1 -cm moderately differentiated cancer (ER+, PR-, HER 2 1+) s/p lumpectomy and SLNbx negative. Oncotype DX 26 Received AC Received XRT • 10/13 Started on letrozole Tolerated well • 12/17 Developed persistent L hip pain. Imaging showed sclerotic lesion in femur, several ribs. Femur bx showed adeno. CA, ER+, PR-, HER 2 -. Tumor markers WNL 1/18 Started on ribociclib + fulvestrant, tolerated well • 4/18 Pain much improved CT shows increased sclerosis of known lesions and two “new” sclerotic rib lesions
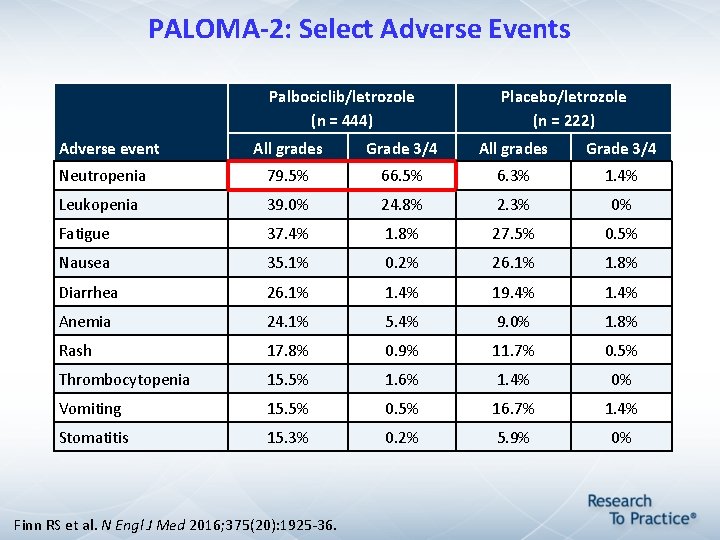
PALOMA-2: Select Adverse Events Palbociclib/letrozole (n = 444) Adverse event Placebo/letrozole (n = 222) All grades Grade 3/4 Neutropenia 79. 5% 66. 5% 6. 3% 1. 4% Leukopenia 39. 0% 24. 8% 2. 3% 0% Fatigue 37. 4% 1. 8% 27. 5% 0. 5% Nausea 35. 1% 0. 2% 26. 1% 1. 8% Diarrhea 26. 1% 1. 4% 19. 4% 1. 4% Anemia 24. 1% 5. 4% 9. 0% 1. 8% Rash 17. 8% 0. 9% 11. 7% 0. 5% Thrombocytopenia 15. 5% 1. 6% 1. 4% 0% Vomiting 15. 5% 0. 5% 16. 7% 1. 4% Stomatitis 15. 3% 0. 2% 5. 9% 0% Finn RS et al. N Engl J Med 2016; 375(20): 1925 -36.
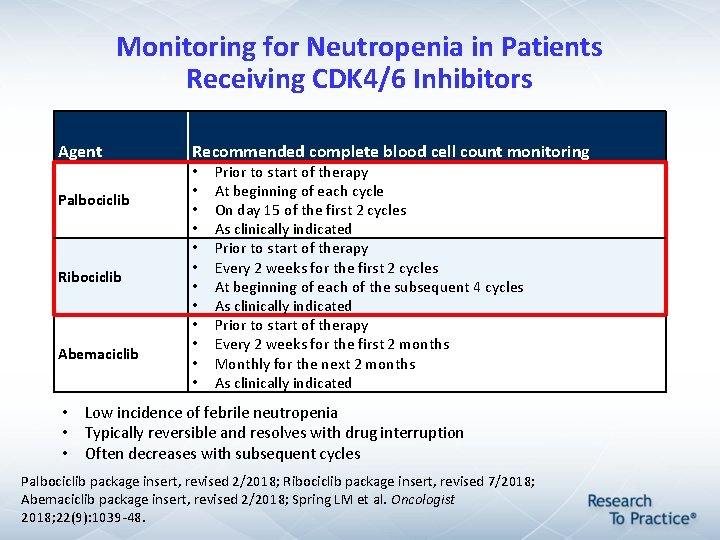
Monitoring for Neutropenia in Patients Receiving CDK 4/6 Inhibitors Agent Palbociclib Ribociclib Abemaciclib Recommended complete blood cell count monitoring • • • Prior to start of therapy At beginning of each cycle On day 15 of the first 2 cycles As clinically indicated Prior to start of therapy Every 2 weeks for the first 2 cycles At beginning of each of the subsequent 4 cycles As clinically indicated Prior to start of therapy Every 2 weeks for the first 2 months Monthly for the next 2 months As clinically indicated • Low incidence of febrile neutropenia • Typically reversible and resolves with drug interruption • Often decreases with subsequent cycles Palbociclib package insert, revised 2/2018; Ribociclib package insert, revised 7/2018; Abemaciclib package insert, revised 2/2018; Spring LM et al. Oncologist 2018; 22(9): 1039 -48.
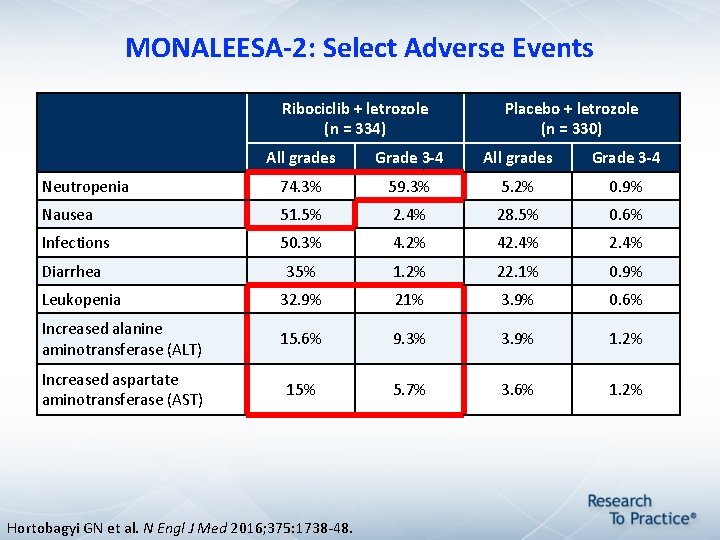
MONALEESA-2: Select Adverse Events Ribociclib + letrozole (n = 334) Placebo + letrozole (n = 330) All grades Grade 3 -4 Neutropenia 74. 3% 59. 3% 5. 2% 0. 9% Nausea 51. 5% 2. 4% 28. 5% 0. 6% Infections 50. 3% 4. 2% 42. 4% Diarrhea 35% 1. 2% 22. 1% 0. 9% Leukopenia 32. 9% 21% 3. 9% 0. 6% Increased alanine aminotransferase (ALT) 15. 6% 9. 3% 3. 9% 1. 2% Increased aspartate aminotransferase (AST) 15% 5. 7% 3. 6% 1. 2% Hortobagyi GN et al. N Engl J Med 2016; 375: 1738 -48.
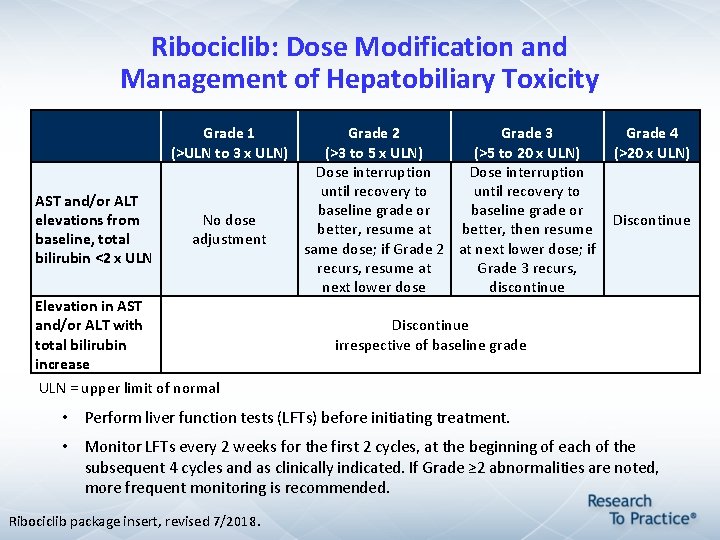
Ribociclib: Dose Modification and Management of Hepatobiliary Toxicity Grade 1 (>ULN to 3 x ULN) AST and/or ALT elevations from baseline, total bilirubin <2 x ULN No dose adjustment Elevation in AST and/or ALT with total bilirubin increase Grade 2 Grade 3 Grade 4 (>3 to 5 x ULN) (>5 to 20 x ULN) (>20 x ULN) Dose interruption until recovery to baseline grade or Discontinue better, resume at better, then resume same dose; if Grade 2 at next lower dose; if recurs, resume at Grade 3 recurs, next lower dose discontinue Discontinue irrespective of baseline grade ULN = upper limit of normal • Perform liver function tests (LFTs) before initiating treatment. • Monitor LFTs every 2 weeks for the first 2 cycles, at the beginning of each of the subsequent 4 cycles and as clinically indicated. If Grade ≥ 2 abnormalities are noted, more frequent monitoring is recommended. Ribociclib package insert, revised 7/2018.
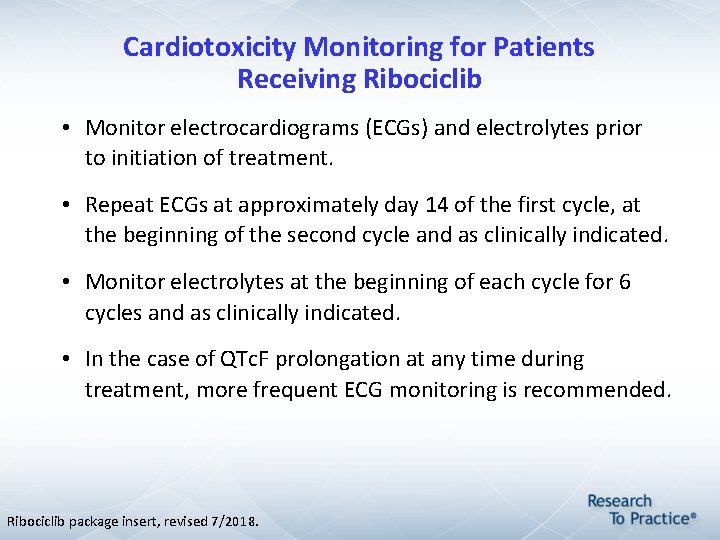
Cardiotoxicity Monitoring for Patients Receiving Ribociclib • Monitor electrocardiograms (ECGs) and electrolytes prior to initiation of treatment. • Repeat ECGs at approximately day 14 of the first cycle, at the beginning of the second cycle and as clinically indicated. • Monitor electrolytes at the beginning of each cycle for 6 cycles and as clinically indicated. • In the case of QTc. F prolongation at any time during treatment, more frequent ECG monitoring is recommended. Ribociclib package insert, revised 7/2018.
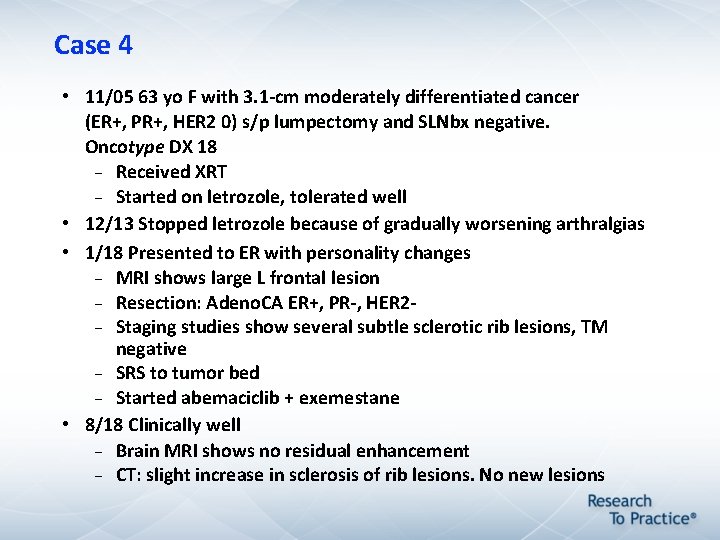
Case 4 • 11/05 63 yo F with 3. 1 -cm moderately differentiated cancer (ER+, PR+, HER 2 0) s/p lumpectomy and SLNbx negative. Oncotype DX 18 Received XRT Started on letrozole, tolerated well • 12/13 Stopped letrozole because of gradually worsening arthralgias • 1/18 Presented to ER with personality changes MRI shows large L frontal lesion Resection: Adeno. CA ER+, PR-, HER 2 Staging studies show several subtle sclerotic rib lesions, TM negative SRS to tumor bed Started abemaciclib + exemestane • 8/18 Clinically well Brain MRI shows no residual enhancement CT: slight increase in sclerosis of rib lesions. No new lesions
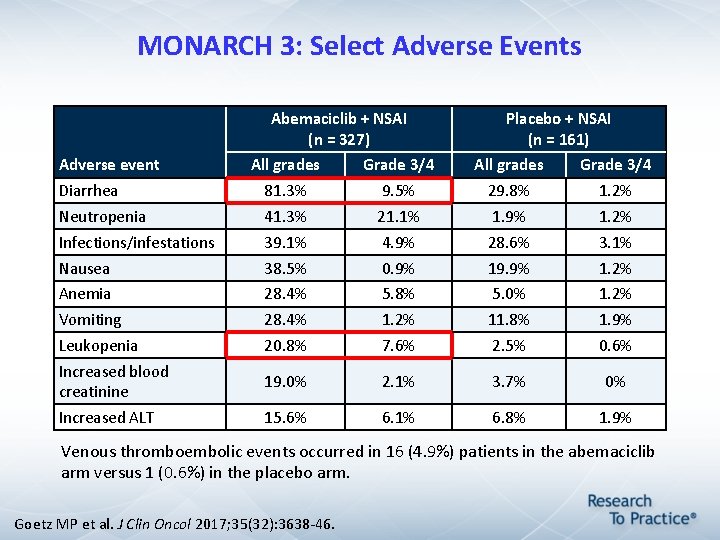
MONARCH 3: Select Adverse Events Abemaciclib + NSAI (n = 327) Adverse event Placebo + NSAI (n = 161) All grades Grade 3/4 Diarrhea 81. 3% 9. 5% 29. 8% 1. 2% Neutropenia 41. 3% 21. 1% 1. 9% 1. 2% Infections/infestations 39. 1% 4. 9% 28. 6% 3. 1% Nausea 38. 5% 0. 9% 19. 9% 1. 2% Anemia 28. 4% 5. 8% 5. 0% 1. 2% Vomiting 28. 4% 1. 2% 11. 8% 1. 9% Leukopenia 20. 8% 7. 6% 2. 5% 0. 6% Increased blood creatinine 19. 0% 2. 1% 3. 7% 0% Increased ALT 15. 6% 6. 1% 6. 8% 1. 9% Venous thromboembolic events occurred in 16 (4. 9%) patients in the abemaciclib arm versus 1 (0. 6%) in the placebo arm. Goetz MP et al. J Clin Oncol 2017; 35(32): 3638 -46.

Strategies to Manage Abemaciclib-Related Gastrointestinal Toxicity • Drink 8 to 10 glasses of water or fluid each day. • Eat small, frequent meals throughout the day rather than a few large meals. • Eat bland, low-fiber foods, such as bananas, applesauce, potatoes, chicken, rice and toast. • Avoid high-fiber foods, such as raw vegetables, raw fruits and whole grains. • Avoid foods that cause gas, such as broccoli and beans. • Avoid lactose-containing foods, such as yogurt and milk. • Avoid spicy, fried and greasy foods. At the first sign of loose stools, patients should start antidiarrheal therapy such as loperamide, increase oral fluids and notify your office. Abemaciclib package insert, revised 2/2018; Hematology/Oncology Pharmacy Association Oral Chemotherapy Education sheet, revised 3/2018.
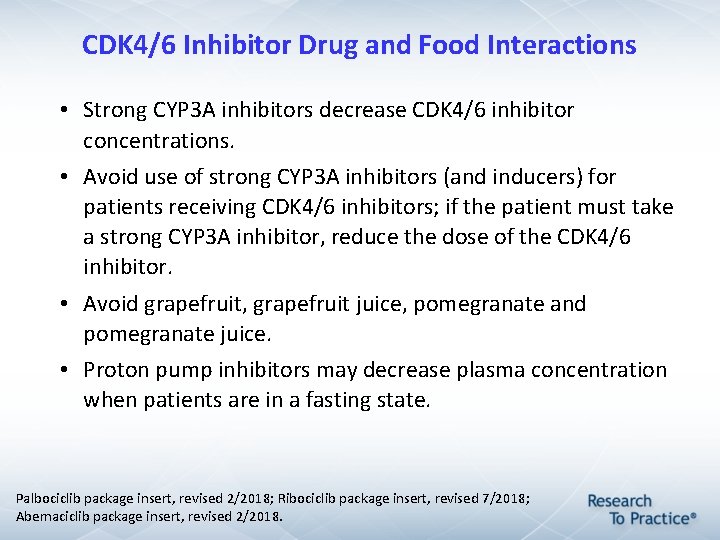
CDK 4/6 Inhibitor Drug and Food Interactions • Strong CYP 3 A inhibitors decrease CDK 4/6 inhibitor concentrations. • Avoid use of strong CYP 3 A inhibitors (and inducers) for patients receiving CDK 4/6 inhibitors; if the patient must take a strong CYP 3 A inhibitor, reduce the dose of the CDK 4/6 inhibitor. • Avoid grapefruit, grapefruit juice, pomegranate and pomegranate juice. • Proton pump inhibitors may decrease plasma concentration when patients are in a fasting state. Palbociclib package insert, revised 2/2018; Ribociclib package insert, revised 7/2018; Abemaciclib package insert, revised 2/2018.
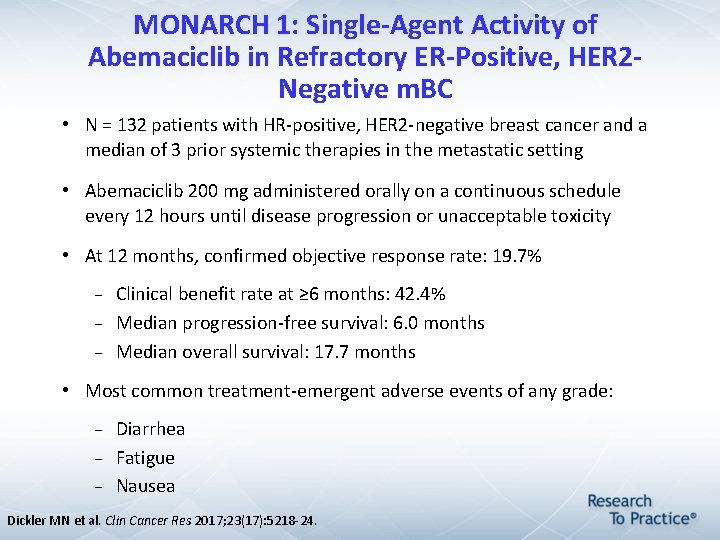
MONARCH 1: Single-Agent Activity of Abemaciclib in Refractory ER-Positive, HER 2 Negative m. BC • N = 132 patients with HR-positive, HER 2 -negative breast cancer and a median of 3 prior systemic therapies in the metastatic setting • Abemaciclib 200 mg administered orally on a continuous schedule every 12 hours until disease progression or unacceptable toxicity • At 12 months, confirmed objective response rate: 19. 7% Clinical benefit rate at ≥ 6 months: 42. 4% Median progression-free survival: 6. 0 months Median overall survival: 17. 7 months • Most common treatment-emergent adverse events of any grade: Diarrhea Fatigue Nausea Dickler MN et al. Clin Cancer Res 2017; 23(17): 5218 -24.
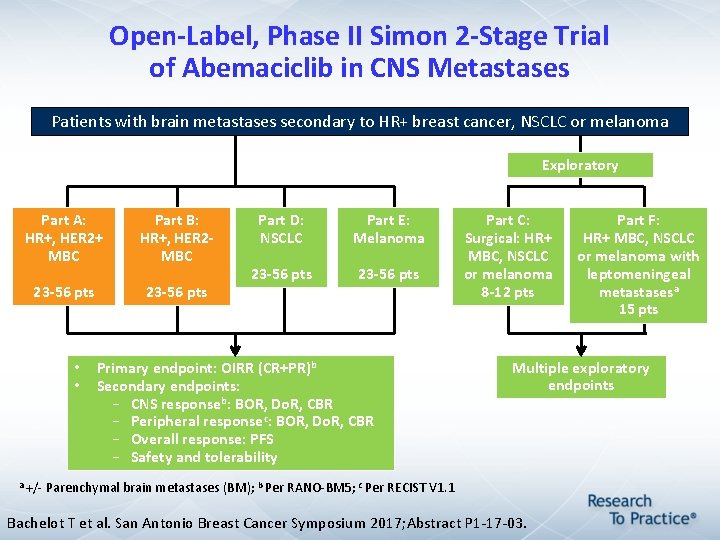
Open-Label, Phase II Simon 2 -Stage Trial of Abemaciclib in CNS Metastases Patients with brain metastases secondary to HR+ breast cancer, NSCLC or melanoma Exploratory Part A: HR+, HER 2+ MBC Part B: HR+, HER 2 - MBC 23 -56 pts • • Part D: NSCLC Part E: Melanoma 23 -56 pts Primary endpoint: OIRR (CR+PR)b Secondary endpoints: – CNS responseb: BOR, Do. R, CBR – Peripheral responsec: BOR, Do. R, CBR – Overall response: PFS – Safety and tolerability Part C: Surgical: HR+ MBC, NSCLC or melanoma 8 -12 pts Part F: HR+ MBC, NSCLC or melanoma with leptomeningeal metastasesa 15 pts Multiple exploratory endpoints a +/- Parenchymal brain metastases (BM); b Per RANO-BM 5; c Per RECIST V 1. 1 Bachelot T et al. San Antonio Breast Cancer Symposium 2017; Abstract P 1 -17 -03.
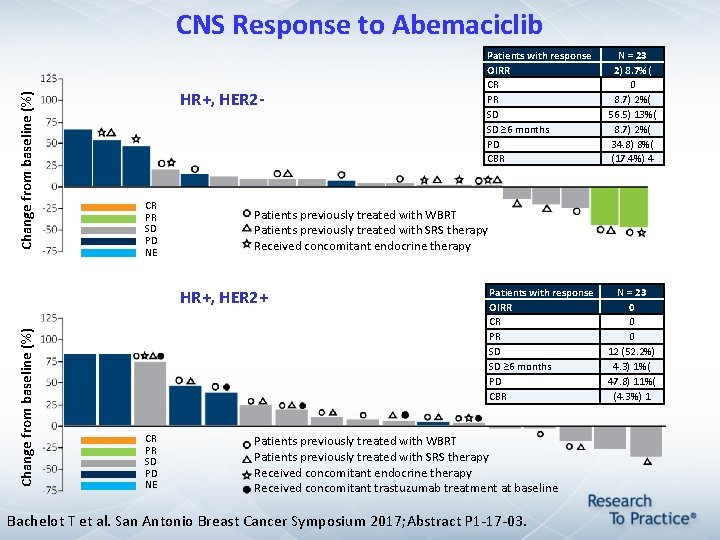
Change from baseline (%) CNS Response to Abemaciclib HR+, HER 2 - CR PR SD PD NE Change from baseline (%) N = 23 2) 8. 7%( 0 8. 7) 2%( 56. 5) 13%( 8. 7) 2%( 34. 8) 8%( (17. 4%) 4 Patients previously treated with WBRT Patients previously treated with SRS therapy Received concomitant endocrine therapy HR+, HER 2+ CR PR SD PD NE Patients with response OIRR CR PR SD SD ≥ 6 months PD CBR Patients previously treated with WBRT Patients previously treated with SRS therapy Received concomitant endocrine therapy Received concomitant trastuzumab treatment at baseline Bachelot T et al. San Antonio Breast Cancer Symposium 2017; Abstract P 1 -17 -03. N = 23 0 0 0 12 (52. 2%) 4. 3) 1%( 47. 8) 11%( (4. 3%) 1
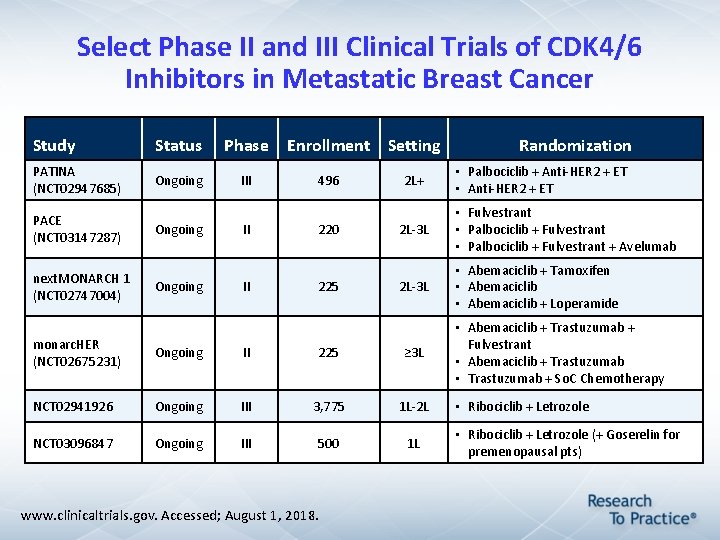
Select Phase II and III Clinical Trials of CDK 4/6 Inhibitors in Metastatic Breast Cancer Study Status Phase PATINA (NCT 02947685) Ongoing III PACE (NCT 03147287) next. MONARCH 1 (NCT 02747004) Ongoing II II Enrollment Setting 496 220 225 2 L+ • Palbociclib + Anti-HER 2 + ET • Anti-HER 2 + ET 2 L-3 L • Fulvestrant • Palbociclib + Fulvestrant + Avelumab 2 L-3 L • Abemaciclib + Tamoxifen • Abemaciclib + Loperamide monarc. HER (NCT 02675231) Ongoing II 225 ≥ 3 L NCT 02941926 Ongoing III 3, 775 1 L-2 L NCT 03096847 Ongoing III 500 1 L www. clinicaltrials. gov. Accessed; August 1, 2018. Randomization • Abemaciclib + Trastuzumab + Fulvestrant • Abemaciclib + Trastuzumab • Trastuzumab + So. C Chemotherapy • Ribociclib + Letrozole (+ Goserelin for premenopausal pts)
Endocrine Treatment of Metastatic Breast Cancer: New Advances; Patient Education Implications Module 1: Estrogen and Progesterone Receptors; Clinical Use of Endocrine Treatment • Incidence, subtypes, staging and treatment • Types of endocrine therapy; response and side effects Module 2: First-Line Endocrine Therapy for Metastatic Disease: Role of CDK 4/6 Inhibitors • CDK 4/6 inhibitors: Overview • CDK 4/6 inhibitors: Efficacy and side effects Module 3: Second-Line Endocrine Therapy for Metastatic Disease: Role of m. TOR Inhibitors • Crosstalk between ER and PI 3 K/AKT/m. TOR signaling pathways • m. TOR inhibitors: Efficacy and side effects Module 4: New Approaches Under Investigation • Triplet therapy: Endocrine therapy + CDK 4/6 inhibitors + m. TOR inhibitors • Efficacy and tolerability of emerging novel agents Module 5: Assessing and Optimizing Treatment Adherence • Scope and clinical implications of adherence • Improving treatment adherence
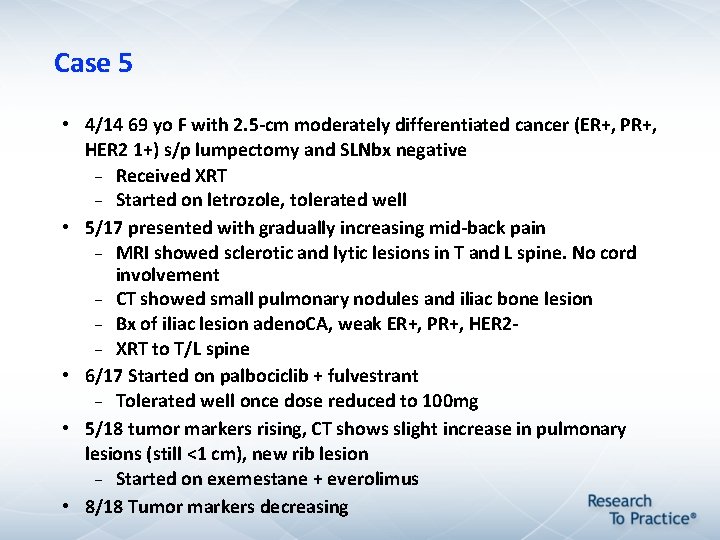
Case 5 • 4/14 69 yo F with 2. 5 -cm moderately differentiated cancer (ER+, PR+, HER 2 1+) s/p lumpectomy and SLNbx negative Received XRT Started on letrozole, tolerated well • 5/17 presented with gradually increasing mid-back pain MRI showed sclerotic and lytic lesions in T and L spine. No cord involvement CT showed small pulmonary nodules and iliac bone lesion Bx of iliac lesion adeno. CA, weak ER+, PR+, HER 2 XRT to T/L spine • 6/17 Started on palbociclib + fulvestrant Tolerated well once dose reduced to 100 mg • 5/18 tumor markers rising, CT shows slight increase in pulmonary lesions (still <1 cm), new rib lesion Started on exemestane + everolimus • 8/18 Tumor markers decreasing
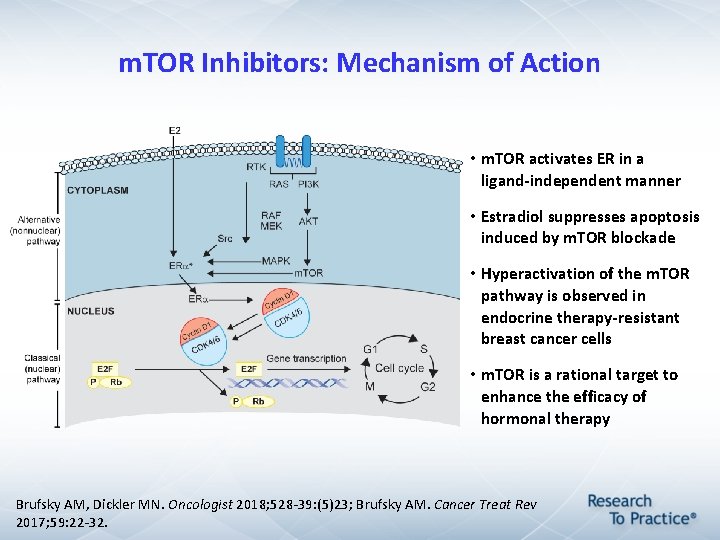
m. TOR Inhibitors: Mechanism of Action • m. TOR activates ER in a ligand-independent manner • Estradiol suppresses apoptosis induced by m. TOR blockade • Hyperactivation of the m. TOR pathway is observed in endocrine therapy-resistant breast cancer cells • m. TOR is a rational target to enhance the efficacy of hormonal therapy Brufsky AM, Dickler MN. Oncologist 2018; 528 -39: (5)23; Brufsky AM. Cancer Treat Rev 2017; 59: 22 -32.
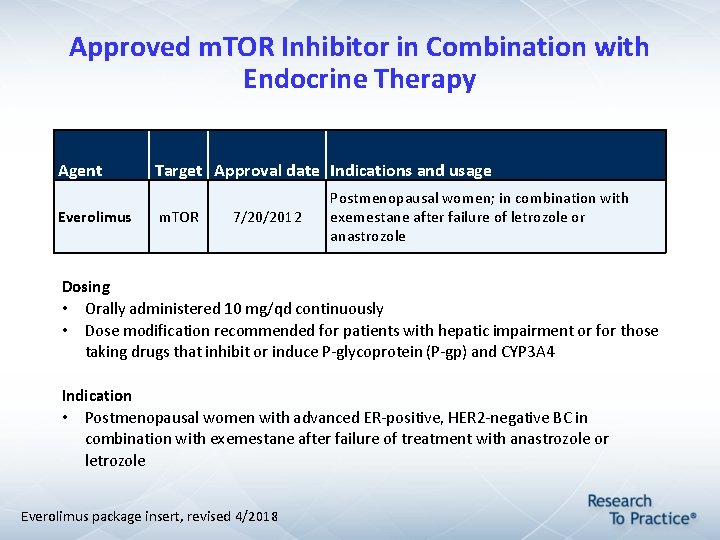
Approved m. TOR Inhibitor in Combination with Endocrine Therapy Agent Everolimus Target Approval date Indications and usage m. TOR 7/20/2012 Postmenopausal women; in combination with exemestane after failure of letrozole or anastrozole Dosing • Orally administered 10 mg/qd continuously • Dose modification recommended for patients with hepatic impairment or for those taking drugs that inhibit or induce P-glycoprotein (P-gp) and CYP 3 A 4 Indication • Postmenopausal women with advanced ER-positive, HER 2 -negative BC in combination with exemestane after failure of treatment with anastrozole or letrozole Everolimus package insert, revised 4/2018

Advances in Therapy 2013; 30: 870 -84.
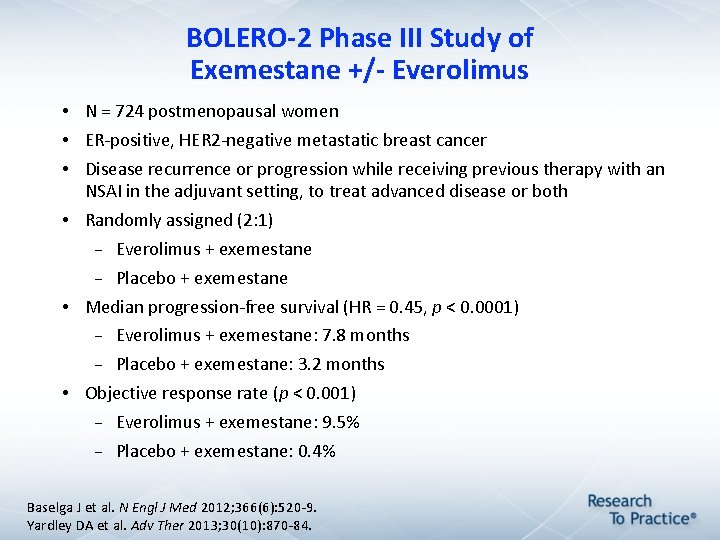
BOLERO-2 Phase III Study of Exemestane +/- Everolimus • N = 724 postmenopausal women • ER-positive, HER 2 -negative metastatic breast cancer • Disease recurrence or progression while receiving previous therapy with an NSAI in the adjuvant setting, to treat advanced disease or both • Randomly assigned (2: 1) Everolimus + exemestane Placebo + exemestane • Median progression-free survival (HR = 0. 45, p < 0. 0001) Everolimus + exemestane: 7. 8 months Placebo + exemestane: 3. 2 months • Objective response rate (p < 0. 001) Everolimus + exemestane: 9. 5% Placebo + exemestane: 0. 4% Baselga J et al. N Engl J Med 2012; 366(6): 520 -9. Yardley DA et al. Adv Ther 2013; 30(10): 870 -84.
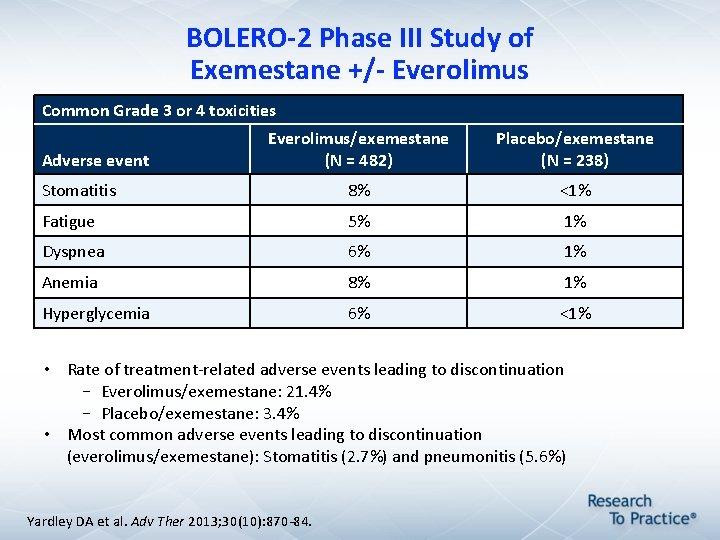
BOLERO-2 Phase III Study of Exemestane +/- Everolimus Common Grade 3 or 4 toxicities Everolimus/exemestane (N = 482) Placebo/exemestane (N = 238) Stomatitis 8% <1% Fatigue 5% 1% Dyspnea 6% 1% Anemia 8% 1% Hyperglycemia 6% <1% Adverse event • Rate of treatment-related adverse events leading to discontinuation – Everolimus/exemestane: 21. 4% – Placebo/exemestane: 3. 4% • Most common adverse events leading to discontinuation (everolimus/exemestane): Stomatitis (2. 7%) and pneumonitis (5. 6%) Yardley DA et al. Adv Ther 2013; 30(10): 870 -84.

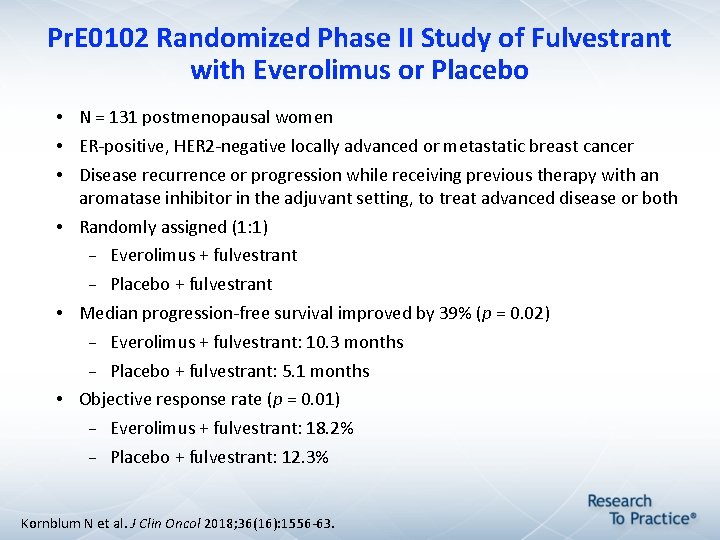
Pr. E 0102 Randomized Phase II Study of Fulvestrant with Everolimus or Placebo • N = 131 postmenopausal women • ER-positive, HER 2 -negative locally advanced or metastatic breast cancer • Disease recurrence or progression while receiving previous therapy with an aromatase inhibitor in the adjuvant setting, to treat advanced disease or both • Randomly assigned (1: 1) Everolimus + fulvestrant Placebo + fulvestrant • Median progression-free survival improved by 39% (p = 0. 02) Everolimus + fulvestrant: 10. 3 months Placebo + fulvestrant: 5. 1 months • Objective response rate (p = 0. 01) Everolimus + fulvestrant: 18. 2% Placebo + fulvestrant: 12. 3% Kornblum N et al. J Clin Oncol 2018; 36(16): 1556 -63.
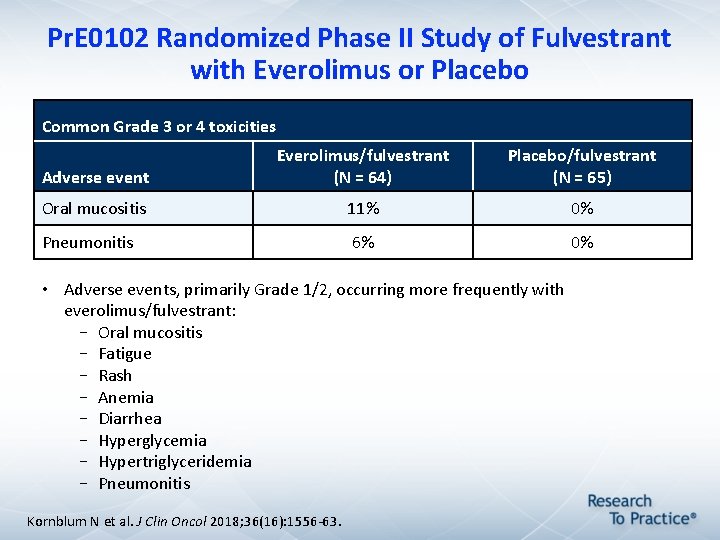
Pr. E 0102 Randomized Phase II Study of Fulvestrant with Everolimus or Placebo Common Grade 3 or 4 toxicities Adverse event Everolimus/fulvestrant (N = 64) Placebo/fulvestrant (N = 65) Oral mucositis 11% 0% Pneumonitis 6% 0% • Adverse events, primarily Grade 1/2, occurring more frequently with everolimus/fulvestrant: – Oral mucositis – Fatigue – Rash – Anemia – Diarrhea – Hyperglycemia – Hypertriglyceridemia – Pneumonitis Kornblum N et al. J Clin Oncol 2018; 36(16): 1556 -63.
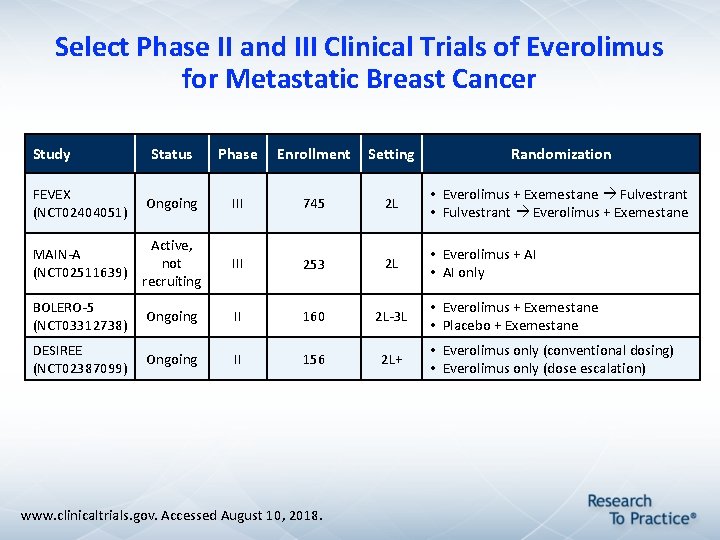
Select Phase II and III Clinical Trials of Everolimus for Metastatic Breast Cancer Study Status Phase Enrollment Setting Randomization FEVEX (NCT 02404051) Ongoing III 745 2 L • Everolimus + Exemestane Fulvestrant • Fulvestrant Everolimus + Exemestane MAIN-A (NCT 02511639) Active, not recruiting III 253 2 L • Everolimus + AI • AI only BOLERO-5 (NCT 03312738) Ongoing II 160 2 L-3 L DESIREE (NCT 02387099) Ongoing II 156 2 L+ www. clinicaltrials. gov. Accessed August 10, 2018. • Everolimus + Exemestane • Placebo + Exemestane • Everolimus only (conventional dosing) • Everolimus only (dose escalation)
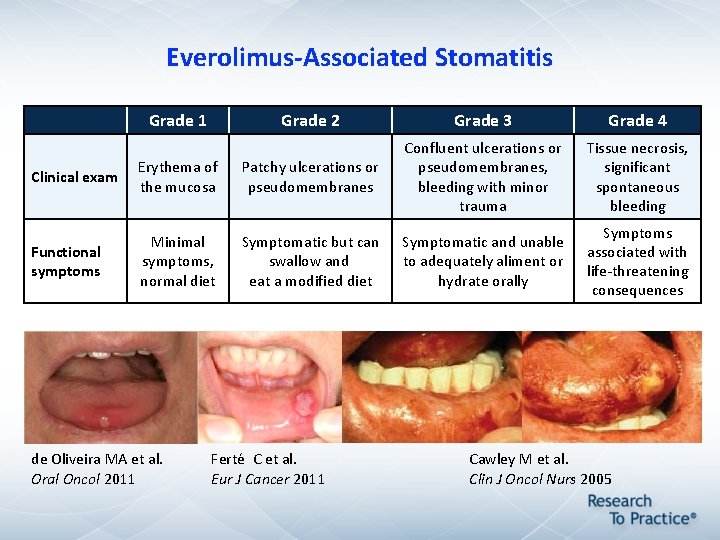
Everolimus-Associated Stomatitis Grade 1 Grade 2 Grade 3 Grade 4 Tissue necrosis, significant spontaneous bleeding Symptoms associated with life-threatening consequences Clinical exam Erythema of the mucosa Patchy ulcerations or pseudomembranes Confluent ulcerations or pseudomembranes, bleeding with minor trauma Functional symptoms Minimal symptoms, normal diet Symptomatic but can swallow and eat a modified diet Symptomatic and unable to adequately aliment or hydrate orally de Oliveira MA et al. Oral Oncol 2011 Ferté C et al. Eur J Cancer 2011 Cawley M et al. Clin J Oncol Nurs 2005
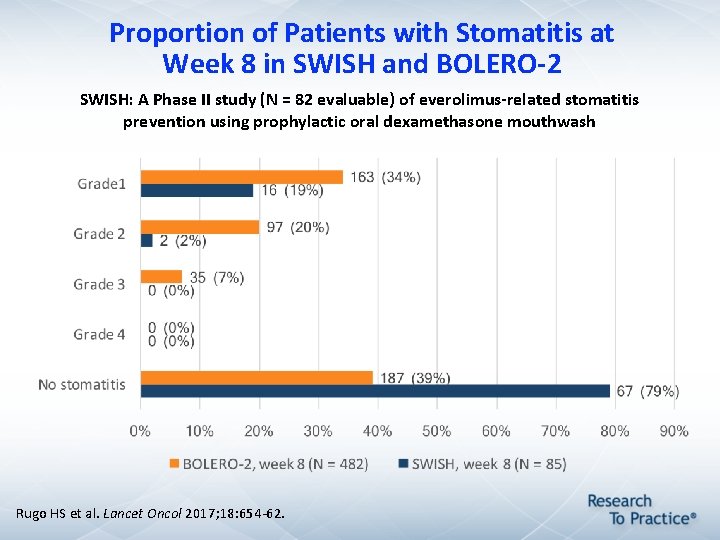
Proportion of Patients with Stomatitis at Week 8 in SWISH and BOLERO-2 SWISH: A Phase II study (N = 82 evaluable) of everolimus-related stomatitis prevention using prophylactic oral dexamethasone mouthwash Rugo HS et al. Lancet Oncol 2017; 18: 654 -62.
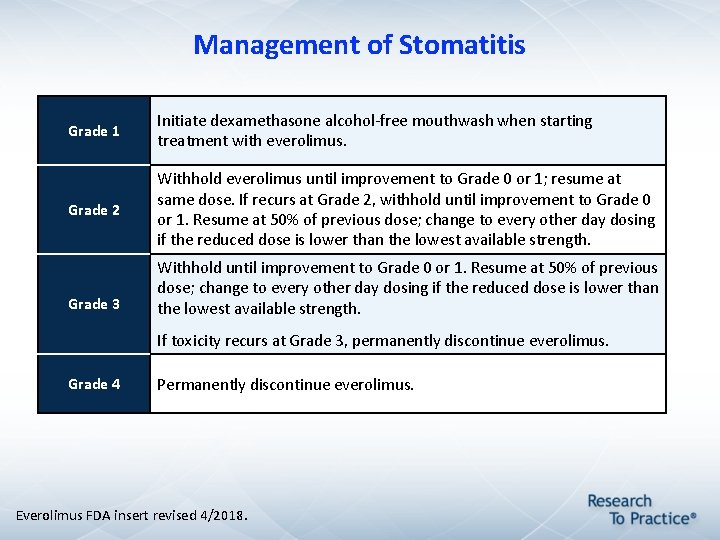
Management of Stomatitis Grade 1 Initiate dexamethasone alcohol-free mouthwash when starting treatment with everolimus. Grade 2 Withhold everolimus until improvement to Grade 0 or 1; resume at same dose. If recurs at Grade 2, withhold until improvement to Grade 0 or 1. Resume at 50% of previous dose; change to every other day dosing if the reduced dose is lower than the lowest available strength. Grade 3 Withhold until improvement to Grade 0 or 1. Resume at 50% of previous dose; change to every other day dosing if the reduced dose is lower than the lowest available strength. If toxicity recurs at Grade 3, permanently discontinue everolimus. Grade 4 Permanently discontinue everolimus. Everolimus FDA insert revised 4/2018.
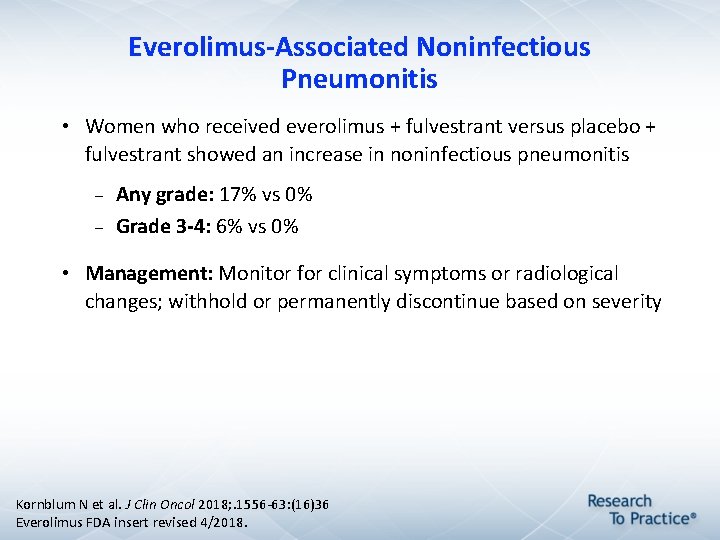
Everolimus-Associated Noninfectious Pneumonitis • Women who received everolimus + fulvestrant versus placebo + fulvestrant showed an increase in noninfectious pneumonitis Any grade: 17% vs 0% Grade 3 -4: 6% vs 0% • Management: Monitor for clinical symptoms or radiological changes; withhold or permanently discontinue based on severity Kornblum N et al. J Clin Oncol 2018; . 1556 -63: (16)36 Everolimus FDA insert revised 4/2018.
Endocrine Treatment of Metastatic Breast Cancer: New Advances; Patient Education Implications Module 1: Estrogen and Progesterone Receptors; Clinical Use of Endocrine Treatment • Incidence, subtypes, staging and treatment • Types of endocrine therapy; response and side effects Module 2: First-Line Endocrine Therapy for Metastatic Disease: Role of CDK 4/6 Inhibitors • CDK 4/6 inhibitors: Overview • CDK 4/6 inhibitors: Efficacy and side effects Module 3: Second-Line Endocrine Therapy for Metastatic Disease: Role of m. TOR Inhibitors • Crosstalk between ER and PI 3 K/AKT/m. TOR signaling pathways • m. TOR inhibitors: Efficacy and side effects Module 4: New Approaches Under Investigation • PI 3 K Inhibitors: Efficacy and side effects • Triplet therapy: Endocrine therapy + CDK 4/6 inhibitors + m. TOR inhibitors • Efficacy and tolerability of emerging novel agents Module 5: Assessing and Optimizing Treatment Adherence • Scope and clinical implications of adherence • Improving treatment adherence

PI 3 K Inhibitor Therapy for ER-Positive Locally Advanced or Metastatic Breast Cancer with a PI 3 K mutation
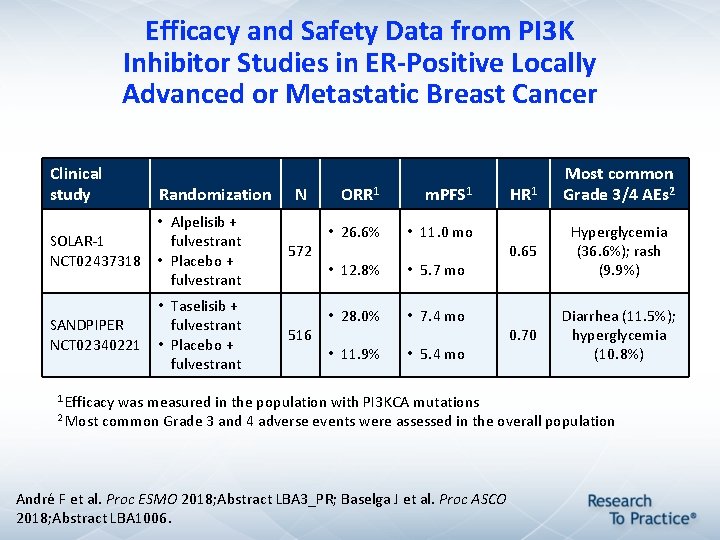
Efficacy and Safety Data from PI 3 K Inhibitor Studies in ER-Positive Locally Advanced or Metastatic Breast Cancer Clinical study Randomization • Alpelisib + SOLAR-1 fulvestrant NCT 02437318 • Placebo + fulvestrant SANDPIPER NCT 02340221 • Taselisib + fulvestrant • Placebo + fulvestrant N 572 516 ORR 1 m. PFS 1 • 26. 6% • 11. 0 mo • 12. 8% • 5. 7 mo • 28. 0% • 7. 4 mo • 11. 9% • 5. 4 mo 1 Efficacy was measured in the population with PI 3 KCA mutations HR 1 Most common Grade 3/4 AEs 2 0. 65 Hyperglycemia (36. 6%); rash (9. 9%) 0. 70 Diarrhea (11. 5%); hyperglycemia (10. 8%) 2 Most common Grade 3 and 4 adverse events were assessed in the overall population André F et al. Proc ESMO 2018; Abstract LBA 3_PR; Baselga J et al. Proc ASCO 2018; Abstract LBA 1006.

Triplet Therapy with ET, CDK 4/6 Inhibitors and PI 3 K or m. TOR Inhibitors
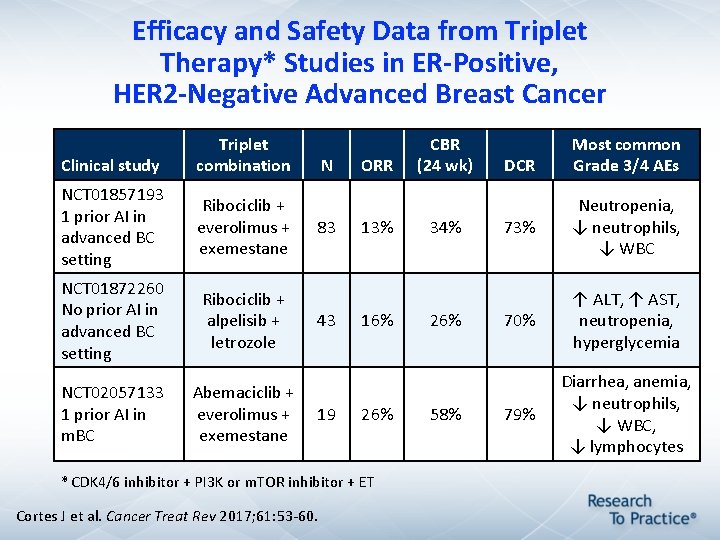
Efficacy and Safety Data from Triplet Therapy* Studies in ER-Positive, HER 2 -Negative Advanced Breast Cancer Clinical study Triplet combination NCT 01857193 1 prior AI in advanced BC setting Ribociclib + everolimus + exemestane NCT 01872260 No prior AI in advanced BC setting Ribociclib + alpelisib + letrozole NCT 02057133 1 prior AI in m. BC Abemaciclib + everolimus + exemestane N 83 43 19 ORR 13% 16% 26% * CDK 4/6 inhibitor + PI 3 K or m. TOR inhibitor + ET Cortes J et al. Cancer Treat Rev 2017; 61: 53 -60. CBR (24 wk) 34% 26% 58% DCR Most common Grade 3/4 AEs 73% Neutropenia, ↓ neutrophils, ↓ WBC 70% ↑ ALT, ↑ AST, neutropenia, hyperglycemia 79% Diarrhea, anemia, ↓ neutrophils, ↓ WBC, ↓ lymphocytes

ER-Positive, HER 2 -Positive Metastatic Breast Cancer
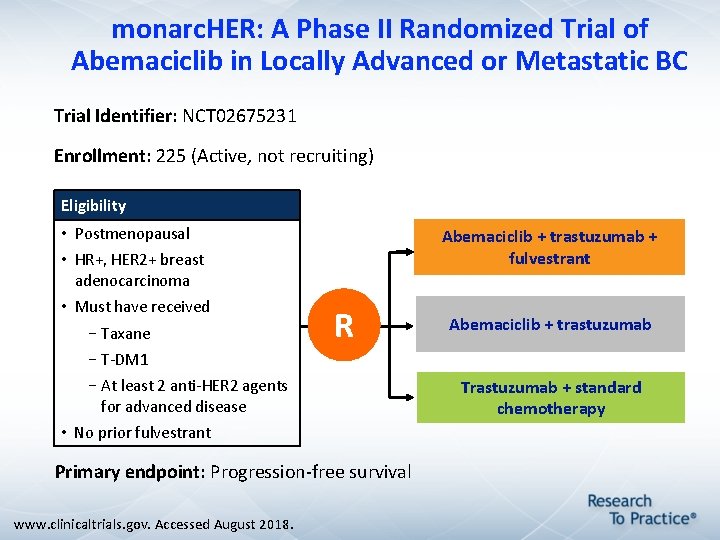
monarc. HER: A Phase II Randomized Trial of Abemaciclib in Locally Advanced or Metastatic BC Trial Identifier: NCT 02675231 Enrollment: 225 (Active, not recruiting) Eligibility • Postmenopausal Abemaciclib + trastuzumab + fulvestrant • HR+, HER 2+ breast adenocarcinoma • Must have received –Taxane R Abemaciclib + trastuzumab –T-DM 1 –At least 2 anti-HER 2 agents for advanced disease • No prior fulvestrant Primary endpoint: Progression-free survival www. clinicaltrials. gov. Accessed August 2018. Trastuzumab + standard chemotherapy
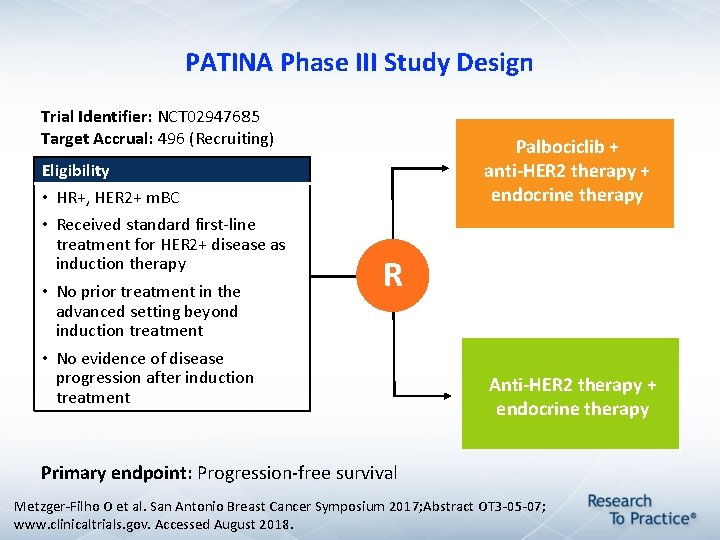
PATINA Phase III Study Design Trial Identifier: NCT 02947685 Target Accrual: 496 (Recruiting) Palbociclib + anti-HER 2 therapy + endocrine therapy Eligibility • HR+, HER 2+ m. BC • Received standard first-line treatment for HER 2+ disease as induction therapy • No prior treatment in the advanced setting beyond induction treatment • No evidence of disease progression after induction treatment R Anti-HER 2 therapy + endocrine therapy Primary endpoint: Progression-free survival Metzger-Filho O et al. San Antonio Breast Cancer Symposium 2017; Abstract OT 3 -05 -07; www. clinicaltrials. gov. Accessed August 2018.
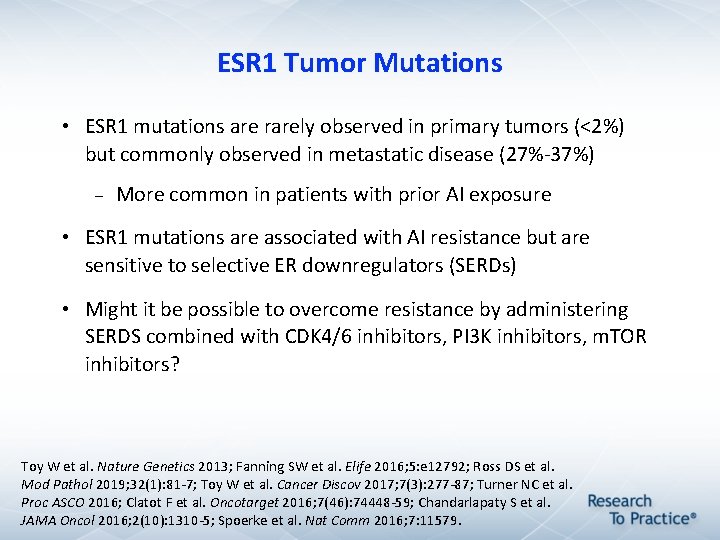
ESR 1 Tumor Mutations • ESR 1 mutations are rarely observed in primary tumors (<2%) but commonly observed in metastatic disease (27%-37%) More common in patients with prior AI exposure • ESR 1 mutations are associated with AI resistance but are sensitive to selective ER downregulators (SERDs) • Might it be possible to overcome resistance by administering SERDS combined with CDK 4/6 inhibitors, PI 3 K inhibitors, m. TOR inhibitors? Toy W et al. Nature Genetics 2013; Fanning SW et al. Elife 2016; 5: e 12792; Ross DS et al. Mod Pathol 2019; 32(1): 81 -7; Toy W et al. Cancer Discov 2017; 7(3): 277 -87; Turner NC et al. Proc ASCO 2016; Clatot F et al. Oncotarget 2016; 7(46): 74448 -59; Chandarlapaty S et al. JAMA Oncol 2016; 2(10): 1310 -5; Spoerke et al. Nat Comm 2016; 7: 11579.
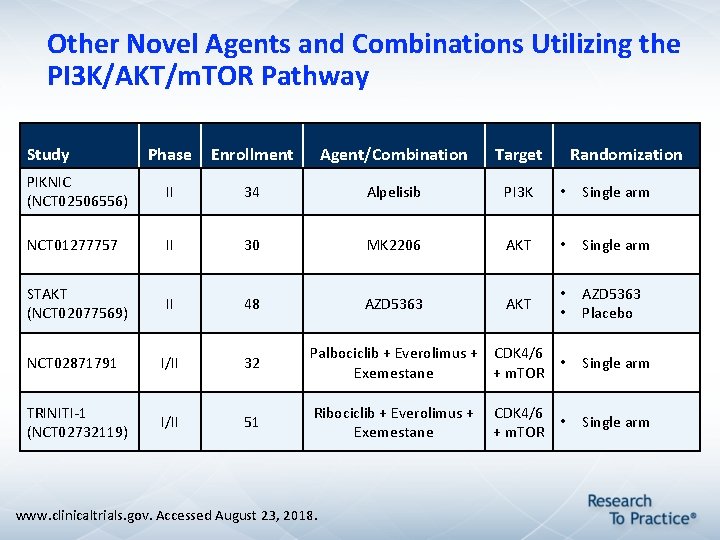
Other Novel Agents and Combinations Utilizing the PI 3 K/AKT/m. TOR Pathway Study Phase Enrollment Agent/Combination Target PIKNIC (NCT 02506556) II 34 Alpelisib PI 3 K • Single arm NCT 01277757 II 30 MK 2206 AKT • Single arm STAKT (NCT 02077569) II 48 AZD 5363 AKT • • AZD 5363 Placebo NCT 02871791 I/II 32 Palbociclib + Everolimus + CDK 4/6 • Exemestane + m. TOR Single arm TRINITI-1 (NCT 02732119) I/II 51 Ribociclib + Everolimus + CDK 4/6 • Exemestane + m. TOR Single arm www. clinicaltrials. gov. Accessed August 23, 2018. Randomization
Endocrine Treatment of Metastatic Breast Cancer: New Advances; Patient Education Implications Module 1: Estrogen and Progesterone Receptors; Clinical Use of Endocrine Treatment • Incidence, subtypes, staging and treatment • Types of endocrine therapy; response and side effects Module 2: First-Line Endocrine Therapy for Metastatic Disease: Role of CDK 4/6 Inhibitors • CDK 4/6 inhibitors: Overview • CDK 4/6 inhibitors: Efficacy and side effects Module 3: Second-Line Endocrine Therapy for Metastatic Disease: Role of m. TOR Inhibitors • Crosstalk between ER and PI 3 K/AKT/m. TOR signaling pathways • m. TOR inhibitors: Efficacy and side effects Module 4: New Approaches Under Investigation • Triplet therapy: Endocrine therapy + CDK 4/6 inhibitors + m. TOR inhibitors • Efficacy and tolerability of emerging novel agents Module 5: Assessing and Optimizing Treatment Adherence • Scope and clinical implications of adherence • Improving treatment adherence
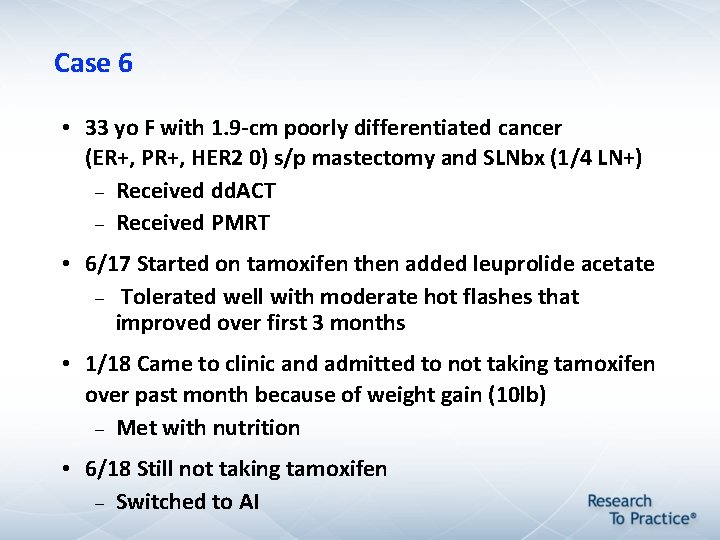
Case 6 • 33 yo F with 1. 9 -cm poorly differentiated cancer (ER+, PR+, HER 2 0) s/p mastectomy and SLNbx (1/4 LN+) Received dd. ACT Received PMRT • 6/17 Started on tamoxifen then added leuprolide acetate Tolerated well with moderate hot flashes that improved over first 3 months • 1/18 Came to clinic and admitted to not taking tamoxifen over past month because of weight gain (10 lb) Met with nutrition • 6/18 Still not taking tamoxifen Switched to AI

Miaskowski C et al. Clin J Oncol Nurs 2008; 12(2): 213 -21.

Adherence to Endocrine Therapy in Clinical Practice “Because breast cancer can be life threatening in the short term, healthcare providers had assumed that all women would be highly motivated to adhere to treatment regimens that would significantly lower their risk for disease recurrence (Waterhouse, Calzone, Mele, & Brenner, 1993). However, many women consider themselves ‘cured’ after initial surgery, chemotherapy, and/or radiotherapy. Such women may fail to recognize the benefits of adjuvant endocrine therapy and take a more relaxed view of the long-term endocrine therapy that follows (Davidson, Vogel, & Wickerham, 2006). ” Miaskowski C et al. Clin J Oncol Nurs 2008; 12(2): 213 -21.
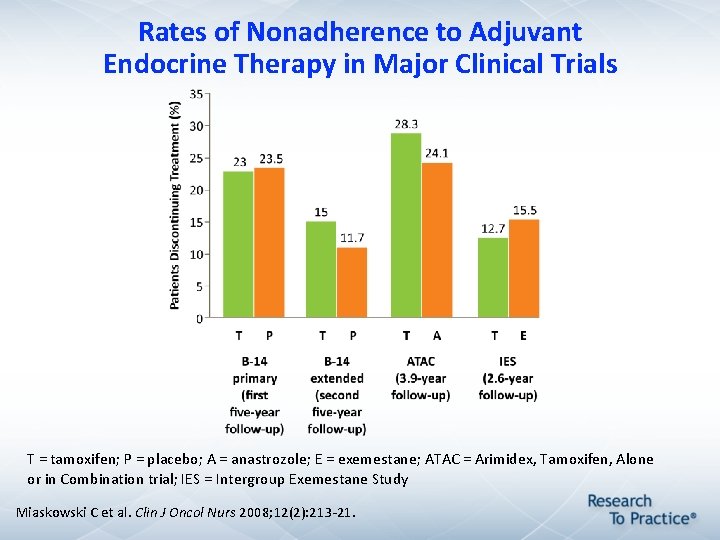
Rates of Nonadherence to Adjuvant Endocrine Therapy in Major Clinical Trials T = tamoxifen; P = placebo; A = anastrozole; E = exemestane; ATAC = Arimidex, Tamoxifen, Alone or in Combination trial; IES = Intergroup Exemestane Study Miaskowski C et al. Clin J Oncol Nurs 2008; 12(2): 213 -21.

Signs and Predictors of Poor Adherence • Patient Chronic disease (long treatment duration) Missed medical appointments Unfilled prescriptions Lack of expected therapeutic response History of poor adherence (eg, to mammography screening) Lack of belief in treatment Lack of support from family and friends Psychological problems, particularly depression • Healthcare professional Complex dosing regimen Inadequate follow-up Poor patient-provider communication • Healthcare system Adverse effects from medication High cost of medication Prescriptions filled at retail pharmacies instead of by mail order Miaskowski C et al. Clin J Oncol Nurs 2008; 12(2): 213 -21.

Modified Morisky Scale • Do you ever forget to take your medication? • Are you careless at times about taking your medication? • When you feel better, do you sometimes stop taking your medication? • Sometimes if you feel worse when you take your medication, do you choose to stop taking it? One point is assigned for each affirmative answer. Patients with scores of 0 or 1 typically show high levels of adherence, whereas those with higher scores are more likely to require interventions to improve adherence. Miaskowski C et al. Clin J Oncol Nurs 2008; 12(2): 213 -21.

Strategies to Help Improve Patient Adherence • Assess the patient’s understanding of the need for the prescribed treatment and its goals. • Provide written information about the treatment. • Review potential side effects of the treatment, and develop management strategies as needed. • Explore the patient’s psychosocial dynamics, including prescription coverage, access to filling prescriptions and support systems. • Provide the patient with professional contact information, especially a phone number, in the event of questions, side effects or concerns. • Schedule follow-up contacts on a regular basis to reassess and reiterate the treatment plan. • Provide a list of organizations and support groups that the patient can consult for more information and advice. Miaskowski C et al. Clin J Oncol Nurs 2008; 12(2): 213 -21.
- Slides: 74