Endo and Exothermic reactions Why do some reactions








- Slides: 12

Endo and Exothermic reactions. Why do some reactions feel hot and some cold? Starter: How do these work? Insert pic of heat pack and ice pack

• Must – I can state that some reactions heat up and some cool down. • Should – I can describe an exothermic and endothermic reaction. • Could – I can use the idea of endothermic and exothermic reactions and link it to real life.

Endo or Exothermic • If a reaction gives out energy it is EXOthermic. A good example is burning. • Think of other words which use exo or ex… • Exoskeleton, external, • Endothermic needs energy for the reaction to occur.

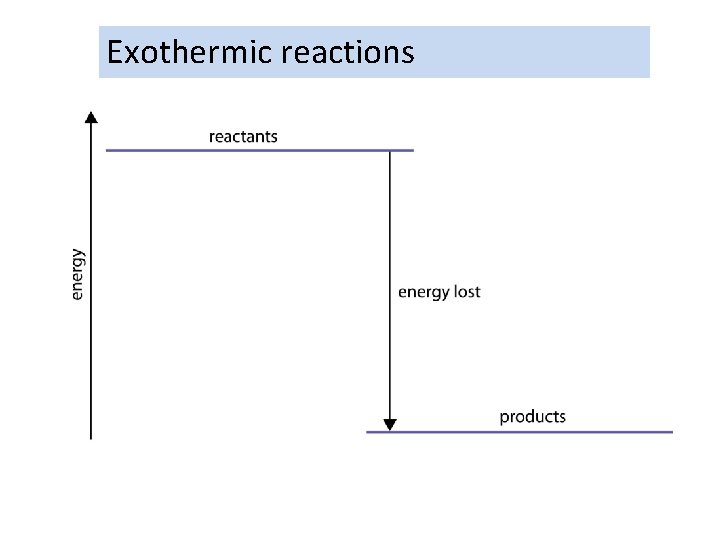
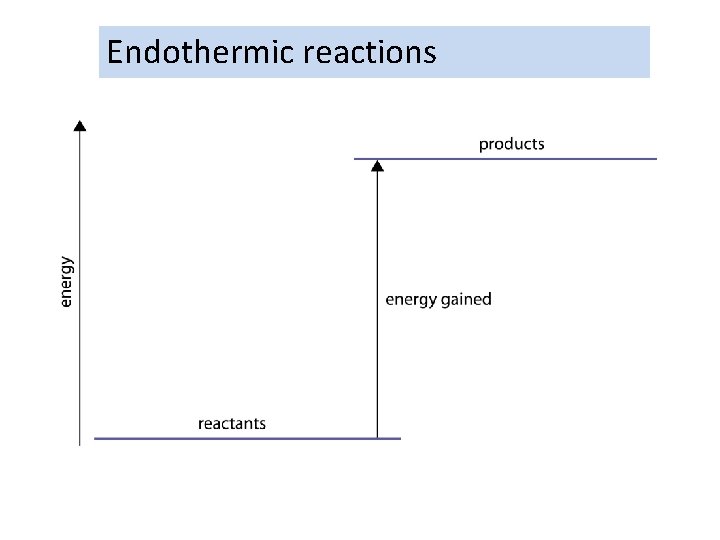
Exothermic and endothermic reactions What are exothermic and endothermic reactions? exothermic reactions release energy – they get hot l ex = out (as in ‘exit’) l thermic = relating to heat endothermic reactions absorb energy – they get cold l en = in (as in ‘entrance’) Most chemical reactions are exothermic.

Reactions require energy to start. The particles have to have enough energy to collide and react. Some need energy to keep going. When started some reactions will give out energy in the form of heat. These are EXOthermic.

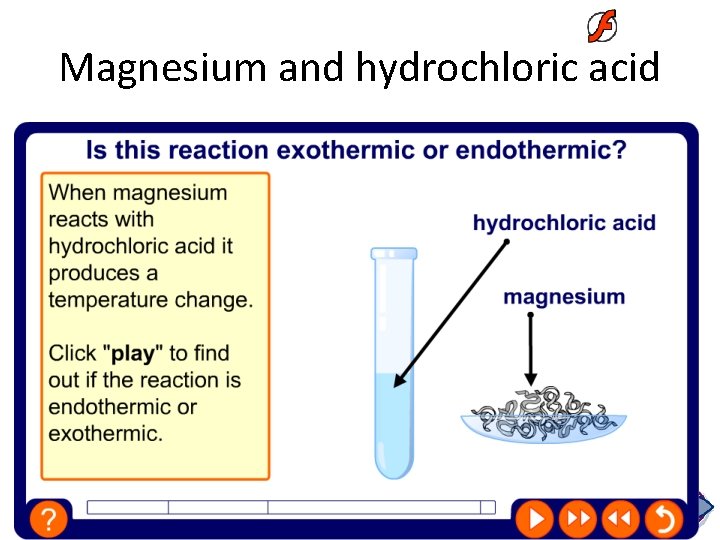
Magnesium and hydrochloric acid

• Some reactions absorb energy from their surroundings. These feel cool to the touch. • They are endothermic.

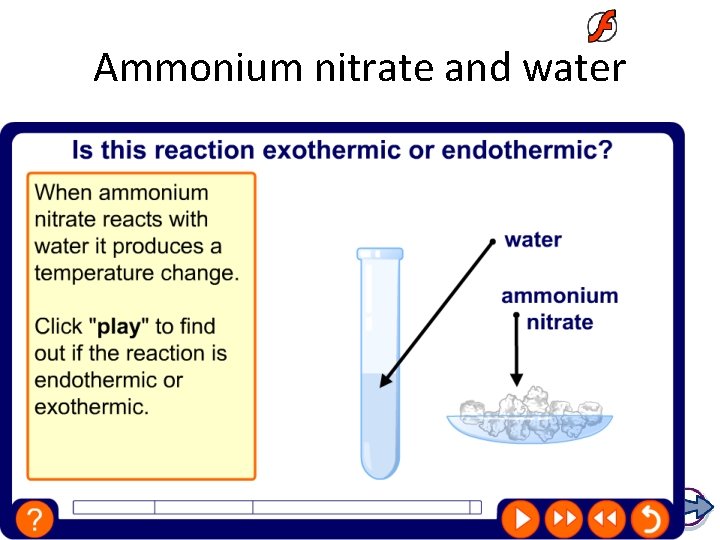
Ammonium nitrate and water

Exothermic reactions

Why do we need to know this ? • 1) Have to know if a reaction is exo/endo to help design appropriate equipment. Endo require heat. • 2) the energy from an exothermic reaction can be used to power the station. • 3) if too much heat is given out, reactions can go faster and get out of control.

Endothermic reactions

Lets investigate. Complete the reactions and find out whether they are exo or endothermic. • Reaction of 10 cm 3 sodium hydroxide solution and 10 cm 3 dilute hydrochloric acid • Reaction of 10 cm 3 sodium hydrogencarbonate solution and 4 small spatulas of citric acid • Reaction of 10 cm 3 copper(II) sulfate solution and 1 small spatula magnesium powder • Reaction of 10 cm 3 sulfuric acid and 3 cm magnesium ribbon