Empirical Formulas Simplest Chemical formula for a compound



- Slides: 13

Empirical Formulas Simplest Chemical formula for a compound

Writing Empirical Formulas 1. Drop the % sign and add g (for grams) to the element 75% Hg and 25% Cl 75 g Hg and 25 g Cl Why can we do this?

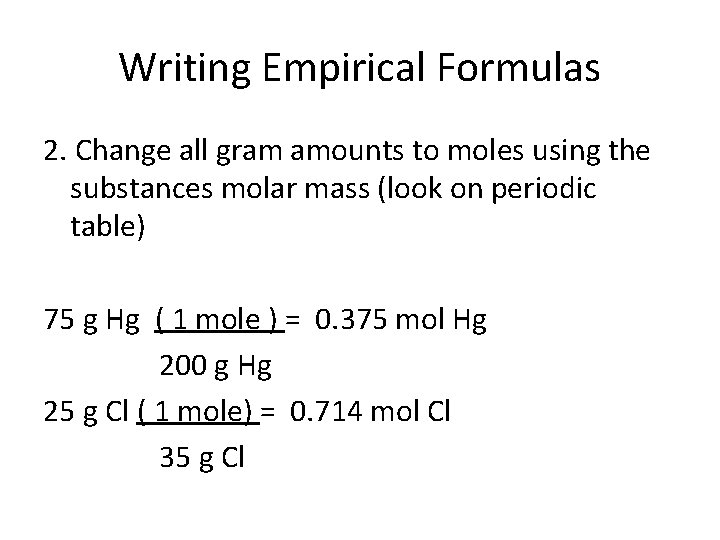
Writing Empirical Formulas 2. Change all gram amounts to moles using the substances molar mass (look on periodic table) 75 g Hg ( 1 mole ) = 0. 375 mol Hg 200 g Hg 25 g Cl ( 1 mole) = 0. 714 mol Cl 35 g Cl

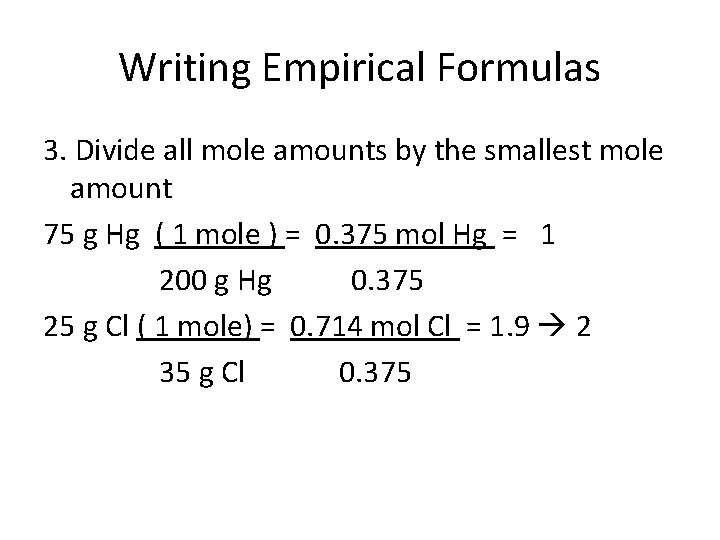
Writing Empirical Formulas 3. Divide all mole amounts by the smallest mole amount 75 g Hg ( 1 mole ) = 0. 375 mol Hg = 1 200 g Hg 0. 375 25 g Cl ( 1 mole) = 0. 714 mol Cl = 1. 9 2 35 g Cl 0. 375

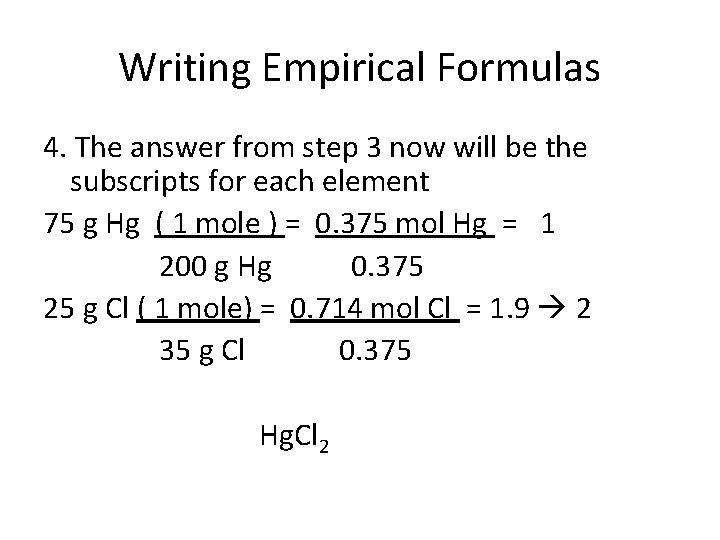
Writing Empirical Formulas 4. The answer from step 3 now will be the subscripts for each element 75 g Hg ( 1 mole ) = 0. 375 mol Hg = 1 200 g Hg 0. 375 25 g Cl ( 1 mole) = 0. 714 mol Cl = 1. 9 2 35 g Cl 0. 375 Hg. Cl 2

Mole Rounding Rules If the mole amount ends in this decimal… • . 25 x 4 • . 33 x 3 • . 5 x 2 • If you multiply you must multiply to all numbers

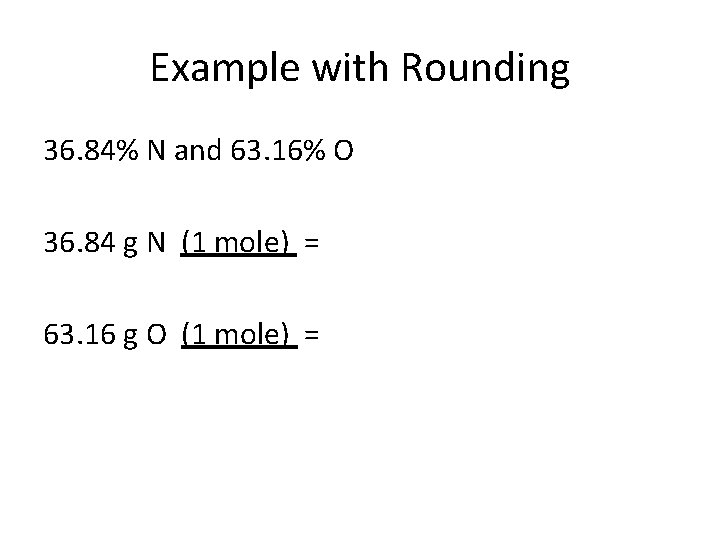
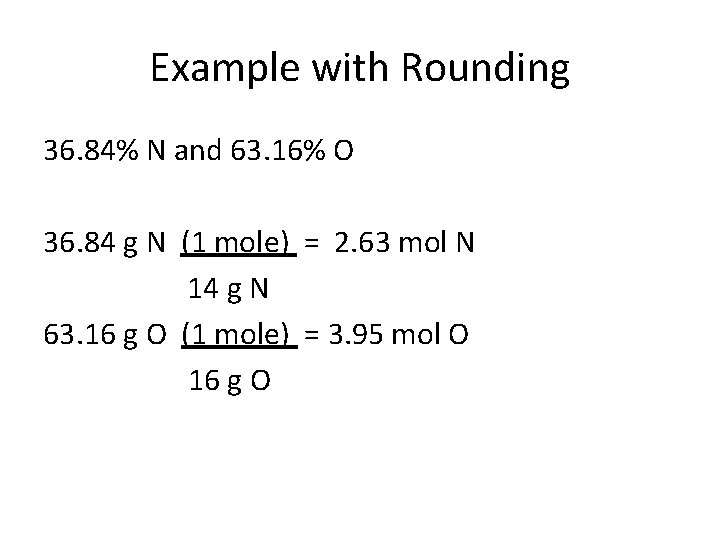
Example with Rounding 36. 84% N and 63. 16% O 36. 84 g N (1 mole) = 63. 16 g O (1 mole) =

Example with Rounding 36. 84% N and 63. 16% O 36. 84 g N (1 mole) = 2. 63 mol N 14 g N 63. 16 g O (1 mole) = 3. 95 mol O 16 g O

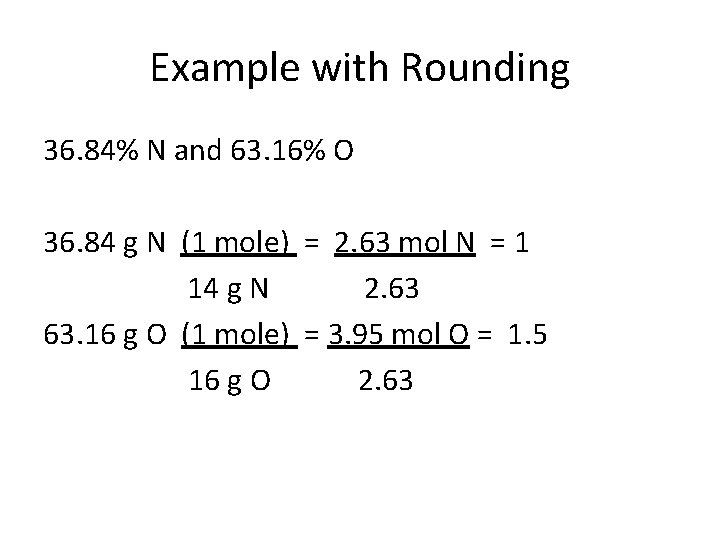
Example with Rounding 36. 84% N and 63. 16% O 36. 84 g N (1 mole) = 2. 63 mol N = 1 14 g N 2. 63 63. 16 g O (1 mole) = 3. 95 mol O = 1. 5 16 g O 2. 63

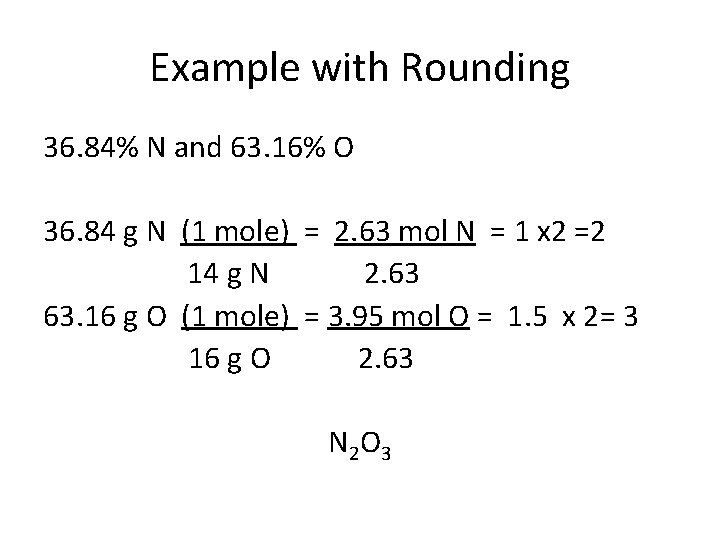
Example with Rounding 36. 84% N and 63. 16% O 36. 84 g N (1 mole) = 2. 63 mol N = 1 x 2 =2 14 g N 2. 63 63. 16 g O (1 mole) = 3. 95 mol O = 1. 5 x 2= 3 16 g O 2. 63 N 2 O 3

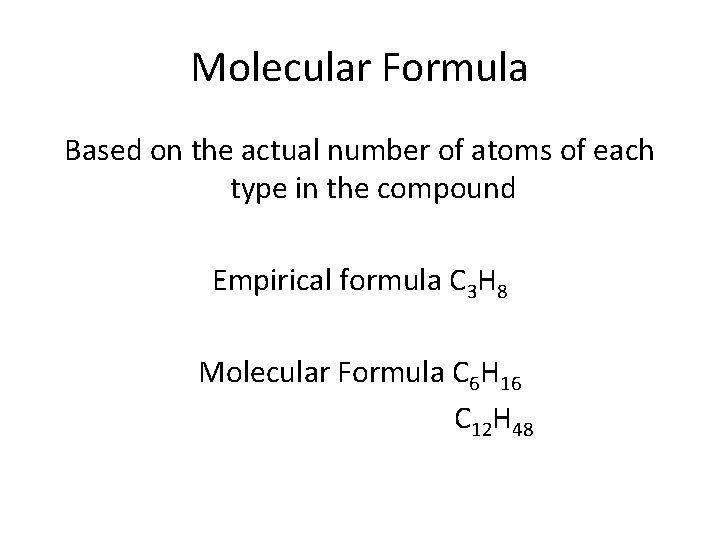
Molecular Formula Based on the actual number of atoms of each type in the compound Empirical formula C 3 H 8 Molecular Formula C 6 H 16 C 12 H 48

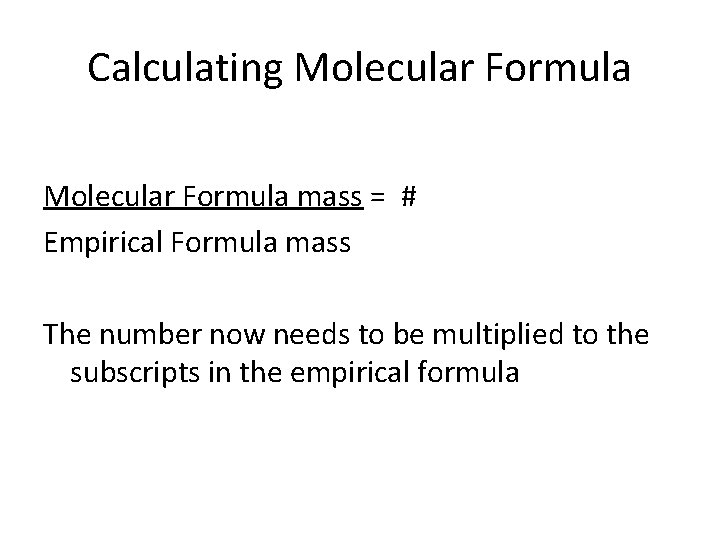
Calculating Molecular Formula mass = # Empirical Formula mass The number now needs to be multiplied to the subscripts in the empirical formula

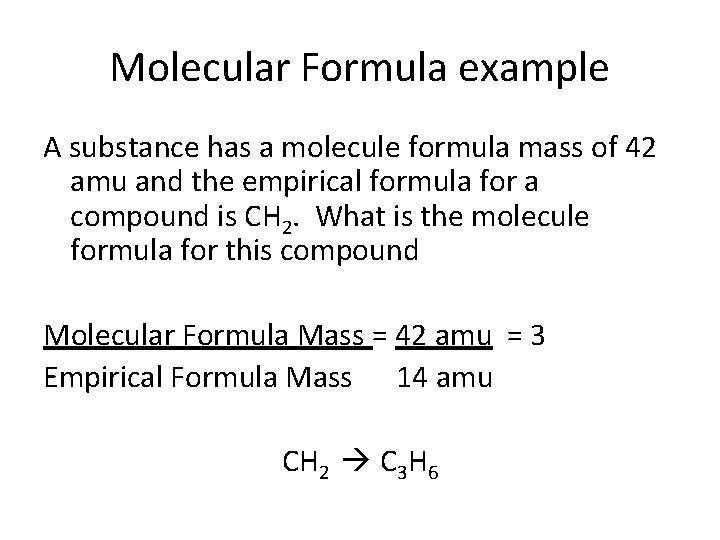
Molecular Formula example A substance has a molecule formula mass of 42 amu and the empirical formula for a compound is CH 2. What is the molecule formula for this compound Molecular Formula Mass = 42 amu = 3 Empirical Formula Mass 14 amu CH 2 C 3 H 6