Empirical Formulas and Molar Mass Part 2 Mass





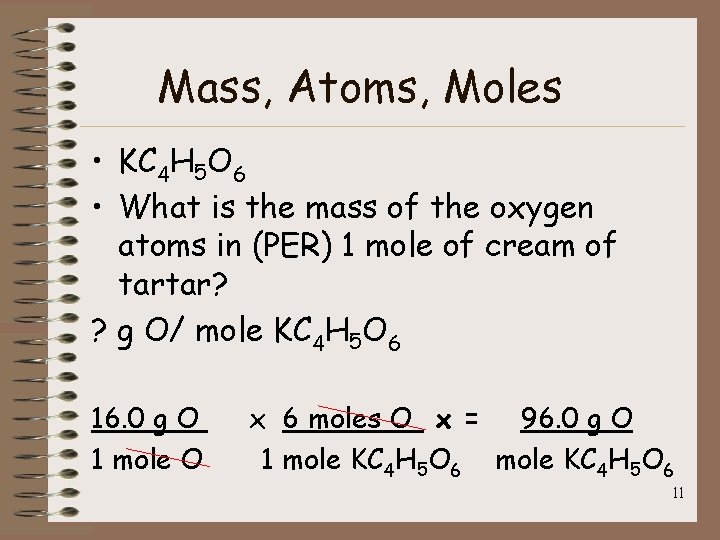
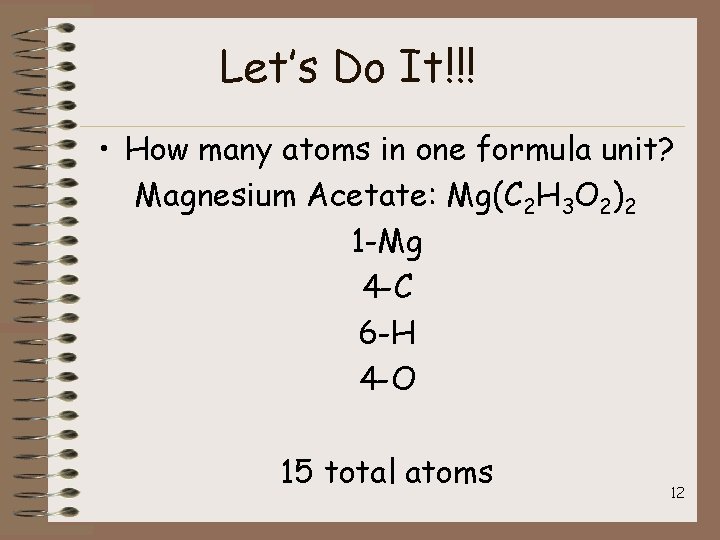

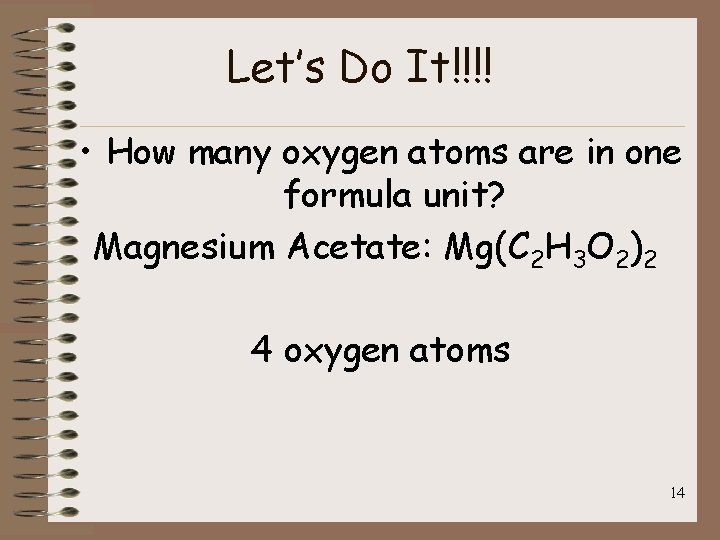
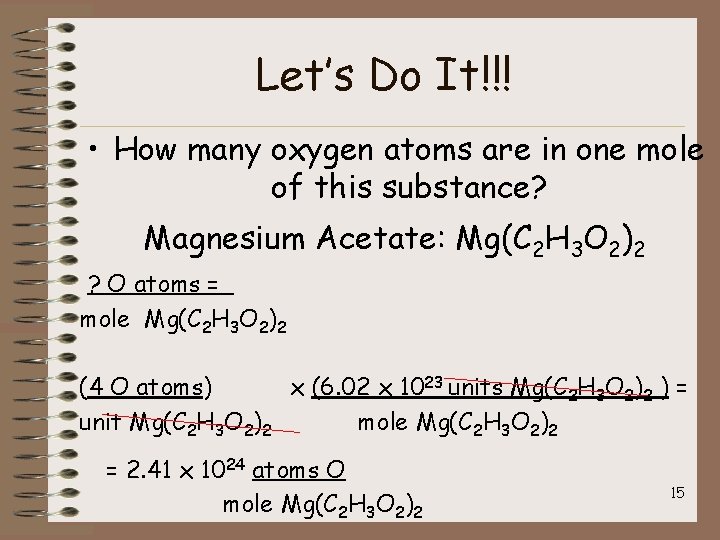
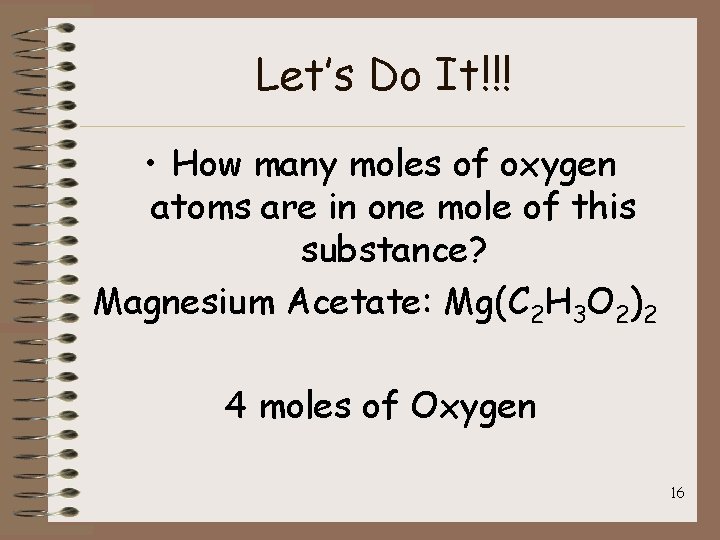
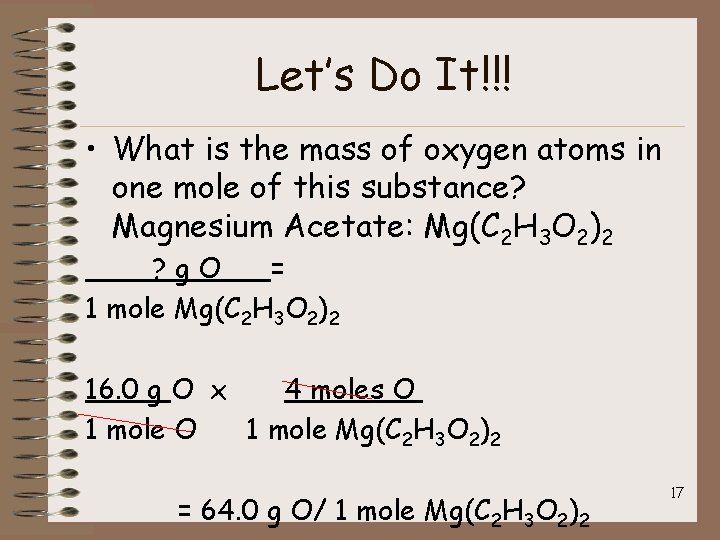

- Slides: 18

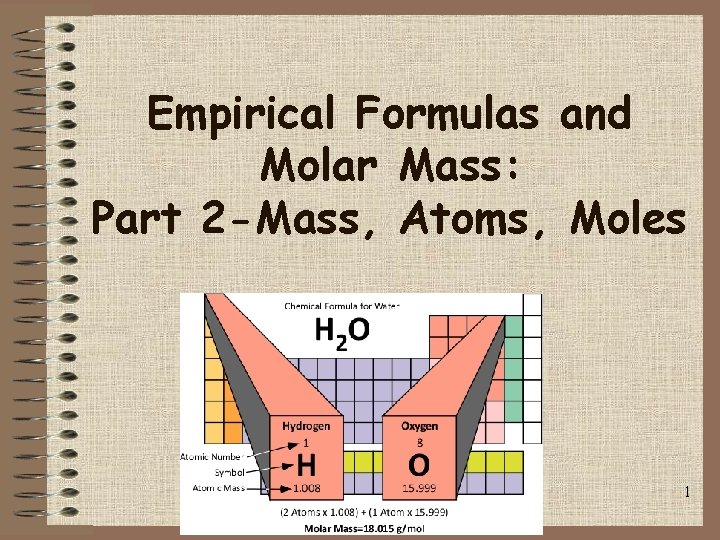
Empirical Formulas and Molar Mass: Part 2 -Mass, Atoms, Moles 1

Objectives • -Be able to use atomic mass to relate grams, moles and atom number • -Determine the number of atoms, moles, and grams of different substances 2

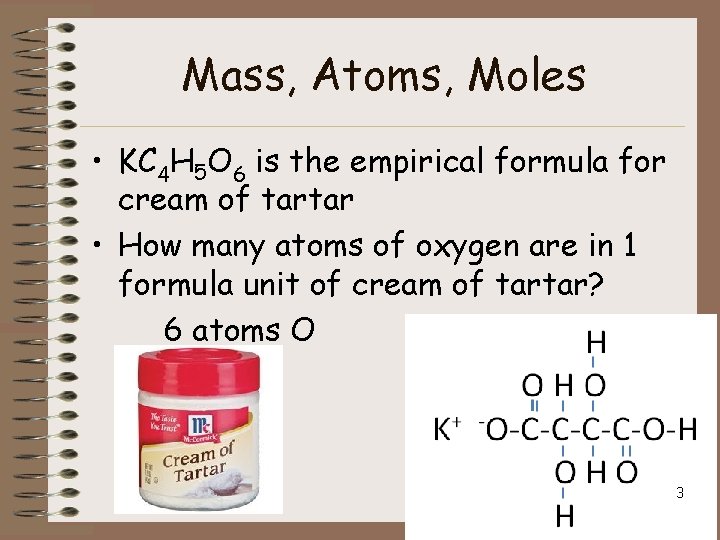
Mass, Atoms, Moles • KC 4 H 5 O 6 is the empirical formula for cream of tartar • How many atoms of oxygen are in 1 formula unit of cream of tartar? 6 atoms O 3

Mass, Atoms, Moles • KC 4 H 5 O 6 • How many atoms are in 1 formula unit of cream of tartar? 16 atoms 1 potassium 4 carbon 5 hydrogen 6 oxygen 4

Mass, Atoms, Moles • KC 4 H 5 O 6 • How many formula units are in 1 mole of cream of tartar? 6. 02 x 1023 formula units 5

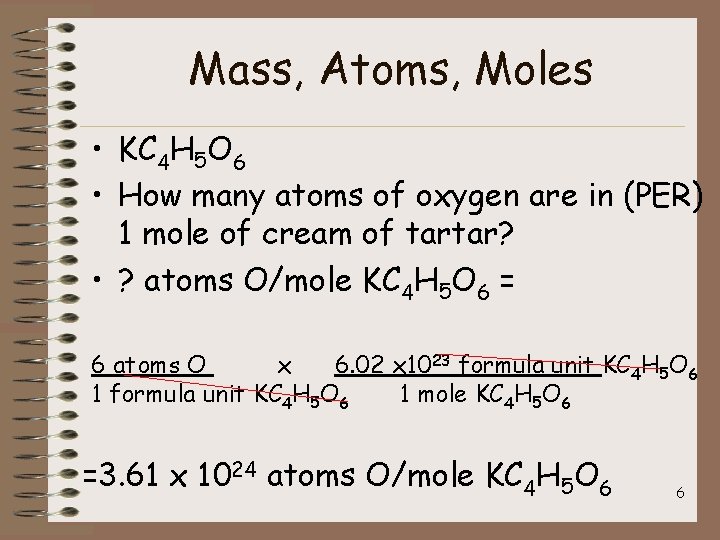
Mass, Atoms, Moles • KC 4 H 5 O 6 • How many atoms of oxygen are in (PER) 1 mole of cream of tartar? • ? atoms O/mole KC 4 H 5 O 6 = 6 atoms O x 6. 02 x 1023 formula unit KC 4 H 5 O 6 1 mole KC 4 H 5 O 6 =3. 61 x 1024 atoms O/mole KC 4 H 5 O 6 6

Mass, Atoms, Moles • KC 4 H 5 O 6 • How many moles of oxygen atoms are in 1 mole of cream of tartar? • Let’s put this in perspective………. 7

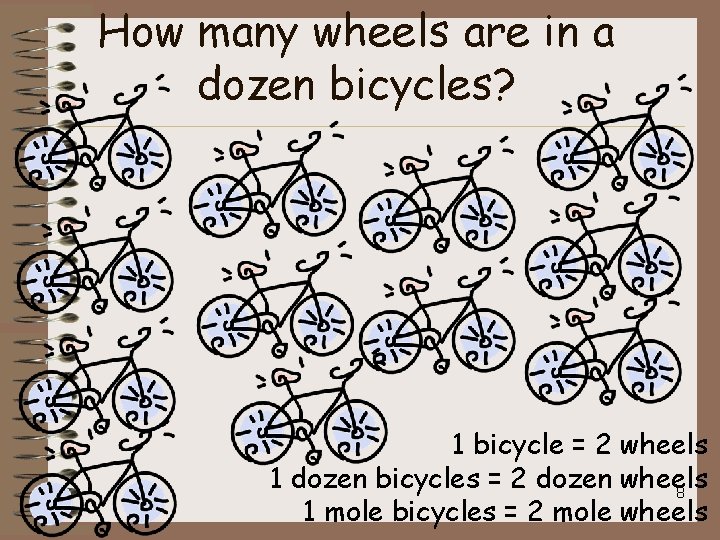
How many wheels are in a dozen bicycles? 1 bicycle = 2 wheels 1 dozen bicycles = 2 dozen wheels 8 1 mole bicycles = 2 mole wheels

How many dozen hydrogen atoms are in 1 dozen water molecules? H 2 O 1 water = 2 hydrogen 1 dozen water = 2 dozen hydrogen 1 mole water = 2 mole hydrogen 9

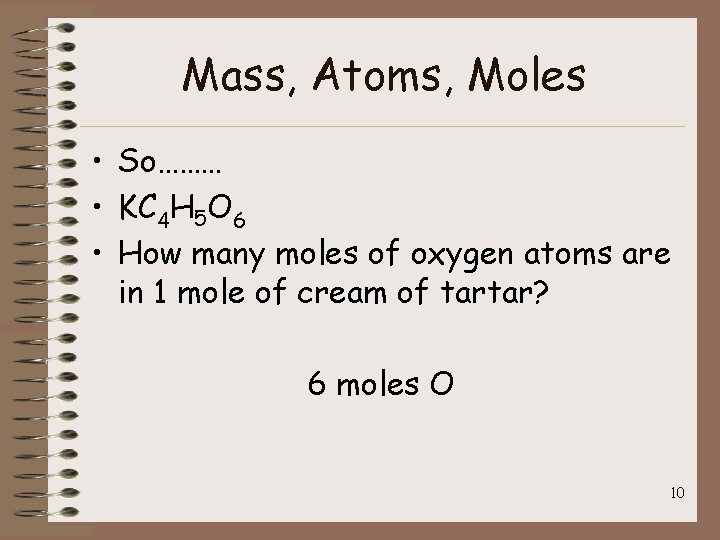
Mass, Atoms, Moles • So……… • KC 4 H 5 O 6 • How many moles of oxygen atoms are in 1 mole of cream of tartar? 6 moles O 10
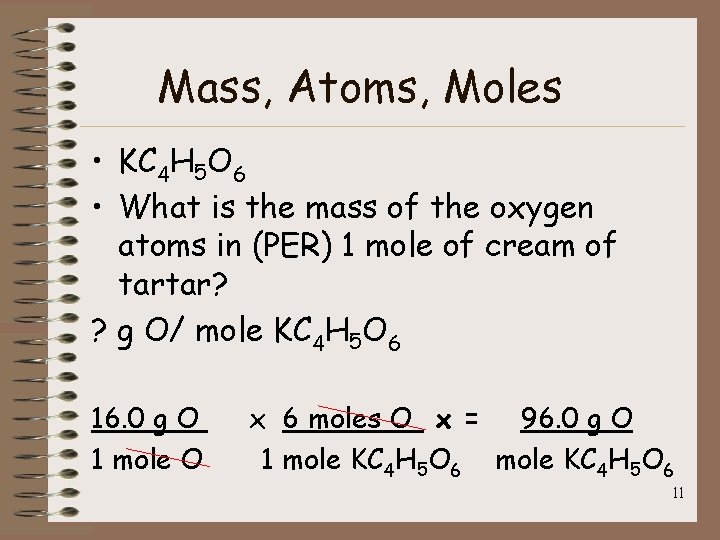
Mass, Atoms, Moles • KC 4 H 5 O 6 • What is the mass of the oxygen atoms in (PER) 1 mole of cream of tartar? ? g O/ mole KC 4 H 5 O 6 16. 0 g O 1 mole O x 6 moles O x = 96. 0 g O 1 mole KC 4 H 5 O 6 11
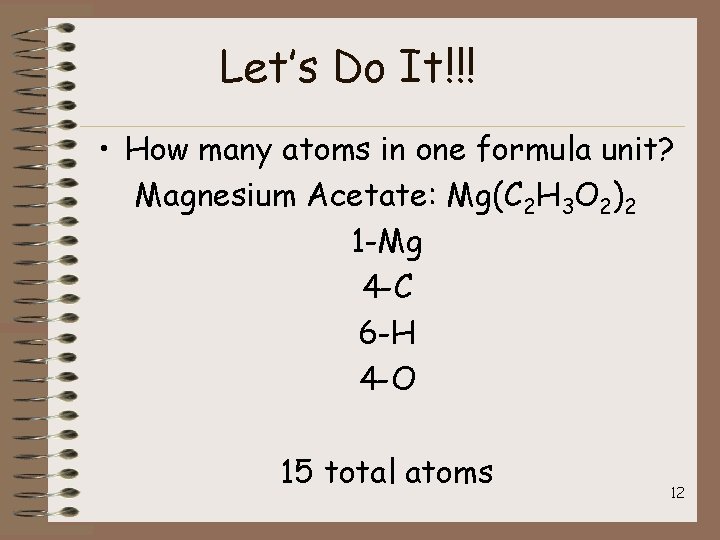
Let’s Do It!!! • How many atoms in one formula unit? Magnesium Acetate: Mg(C 2 H 3 O 2)2 1 -Mg 4 -C 6 -H 4 -O 15 total atoms 12

Let’s Do It!!! • How many formula units in one mole of Magnesium Acetate: Mg(C 2 H 3 O 2)2 6. 02 x 1023 formula units 13
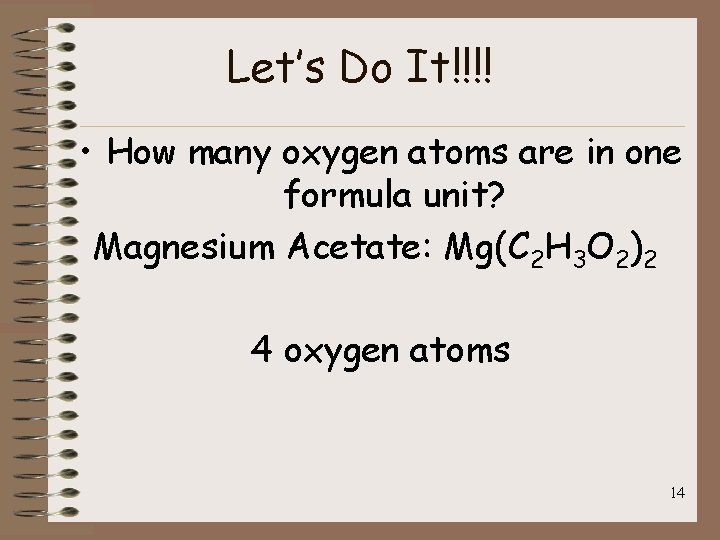
Let’s Do It!!!! • How many oxygen atoms are in one formula unit? Magnesium Acetate: Mg(C 2 H 3 O 2)2 4 oxygen atoms 14
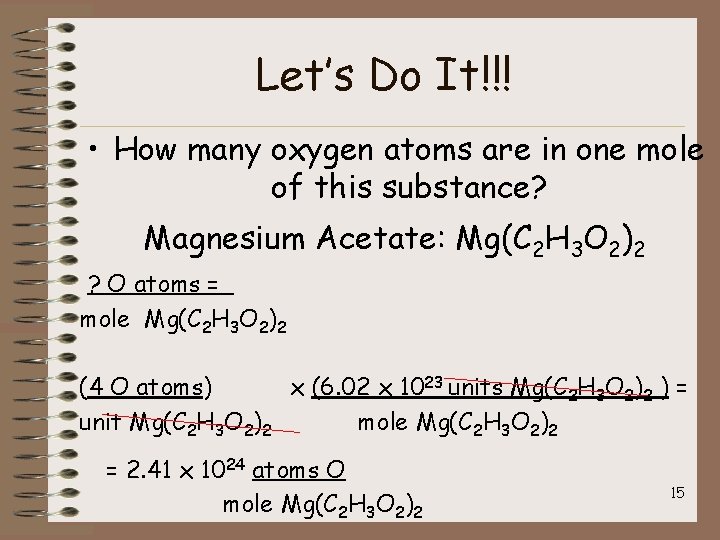
Let’s Do It!!! • How many oxygen atoms are in one mole of this substance? Magnesium Acetate: Mg(C 2 H 3 O 2)2 ? O atoms = mole Mg(C 2 H 3 O 2)2 (4 O atoms) x (6. 02 x 1023 units Mg(C 2 H 3 O 2)2 ) = unit Mg(C 2 H 3 O 2)2 mole Mg(C 2 H 3 O 2)2 = 2. 41 x 1024 atoms O mole Mg(C 2 H 3 O 2)2 15
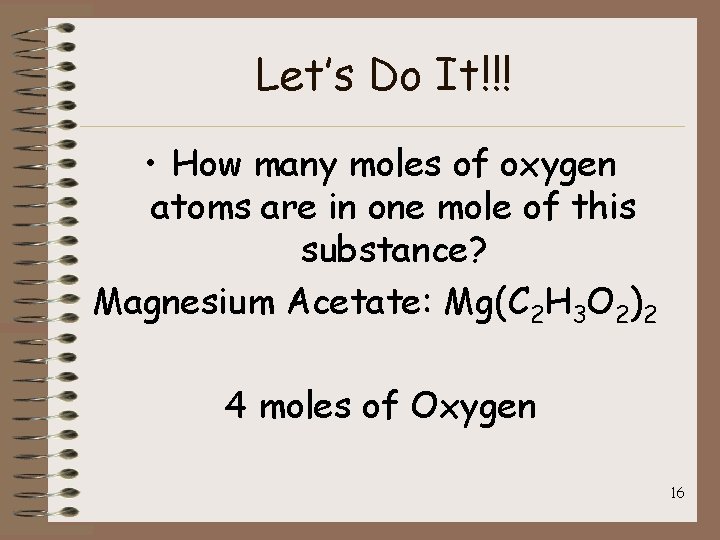
Let’s Do It!!! • How many moles of oxygen atoms are in one mole of this substance? Magnesium Acetate: Mg(C 2 H 3 O 2)2 4 moles of Oxygen 16
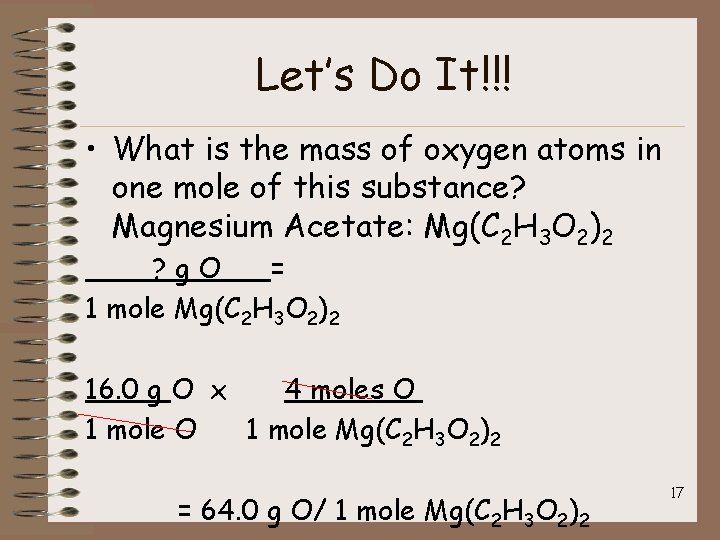
Let’s Do It!!! • What is the mass of oxygen atoms in one mole of this substance? Magnesium Acetate: Mg(C 2 H 3 O 2)2 ? g. O = 1 mole Mg(C 2 H 3 O 2)2 16. 0 g O x 4 moles O 1 mole Mg(C 2 H 3 O 2)2 = 64. 0 g O/ 1 mole Mg(C 2 H 3 O 2)2 17

Objectives • -Be able to use atomic mass to relate grams, moles and atom number • -Determine the number of atoms, moles, and grams of different substances 18