Empirical Formula Empirical based on observation and experiment

Empirical Formula Empirical: based on observation and experiment
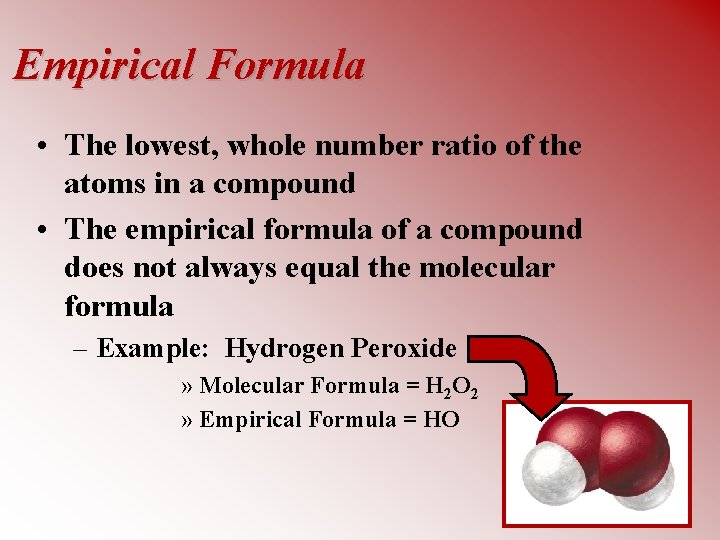
Empirical Formula • The lowest, whole number ratio of the atoms in a compound • The empirical formula of a compound does not always equal the molecular formula – Example: Hydrogen Peroxide » Molecular Formula = H 2 O 2 » Empirical Formula = HO
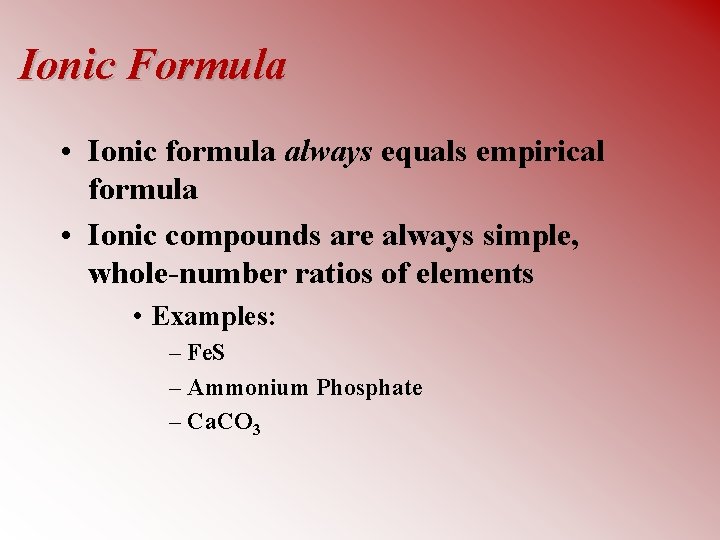
Ionic Formula • Ionic formula always equals empirical formula • Ionic compounds are always simple, whole-number ratios of elements • Examples: – Fe. S – Ammonium Phosphate – Ca. CO 3

Determining Empirical Formula • Example: A compound has a percent composition of 27. 29% carbon and 72. 71% oxygen. What is the compound’s empirical formula?
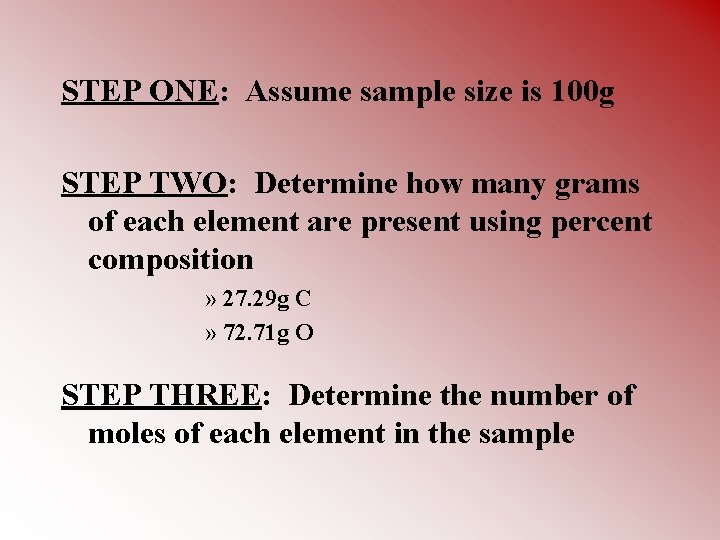
STEP ONE: Assume sample size is 100 g STEP TWO: Determine how many grams of each element are present using percent composition » 27. 29 g C » 72. 71 g O STEP THREE: Determine the number of moles of each element in the sample
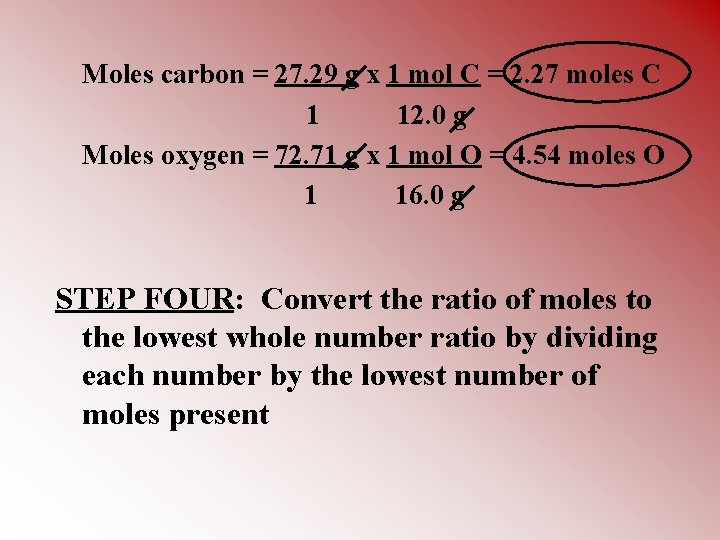
Moles carbon = 27. 29 g x 1 mol C = 2. 27 moles C 1 12. 0 g Moles oxygen = 72. 71 g x 1 mol O = 4. 54 moles O 1 16. 0 g STEP FOUR: Convert the ratio of moles to the lowest whole number ratio by dividing each number by the lowest number of moles present
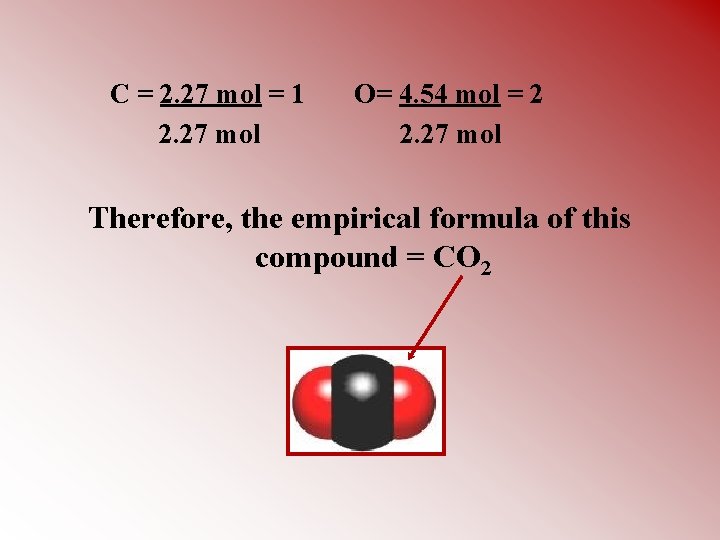
C = 2. 27 mol = 1 2. 27 mol O= 4. 54 mol = 2 2. 27 mol Therefore, the empirical formula of this compound = CO 2
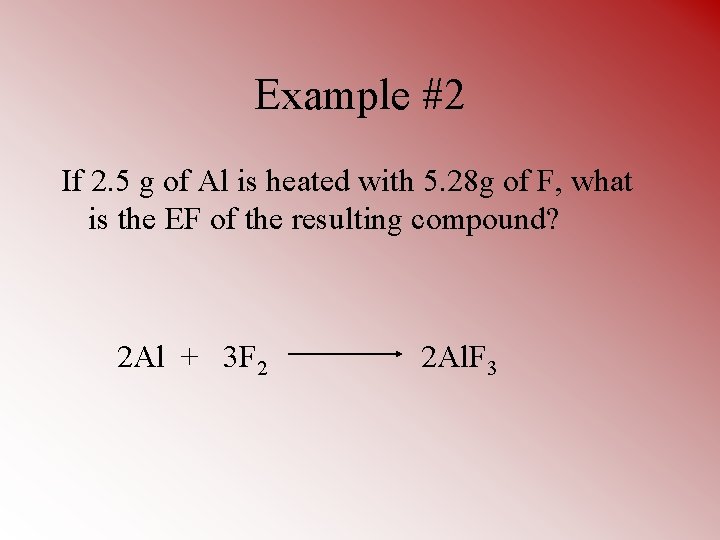
Example #2 If 2. 5 g of Al is heated with 5. 28 g of F, what is the EF of the resulting compound? 2 Al + 3 F 2 2 Al. F 3
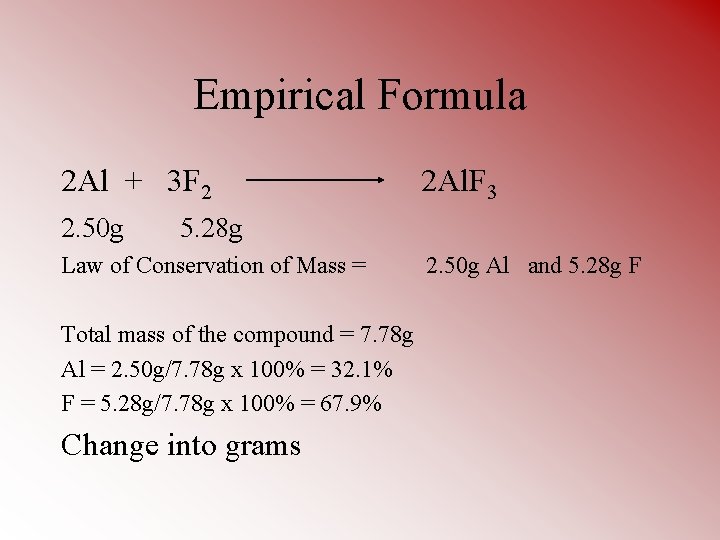
Empirical Formula 2 Al + 3 F 2 2. 50 g 2 Al. F 3 5. 28 g Law of Conservation of Mass = Total mass of the compound = 7. 78 g Al = 2. 50 g/7. 78 g x 100% = 32. 1% F = 5. 28 g/7. 78 g x 100% = 67. 9% Change into grams 2. 50 g Al and 5. 28 g F

Al 32. 1% F 67. 9% 32. 1 g 67. 9 g Determine how many moles of each you have Al F
Molecular Formula • Either the same as empirical formula or a simple, whole number multiple of its empirical formula • Example: Benzene » Empirical = CH » Molecular = C 6 H 6 • Example: Methanol » Empirical = CH 4 O » Molecular = CH 4 O
Determining Molecular Formula • From empirical formula, empirical formula mass (efm) can be determined • Example: HO = 17. 0 g/mol • Molar mass is determined experimentally • Example: 34. 0 g/mol • Number of empirical formula units can be determined by these two values Molar Mass = Empirical Formula Multiplier efm
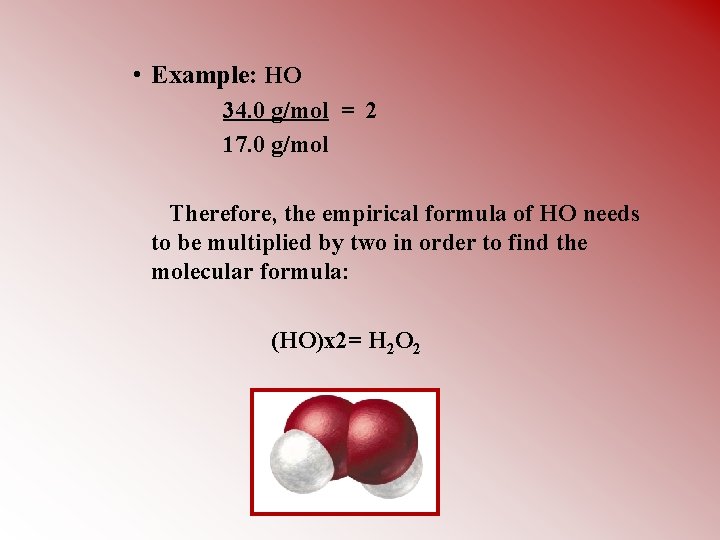
• Example: HO 34. 0 g/mol = 2 17. 0 g/mol Therefore, the empirical formula of HO needs to be multiplied by two in order to find the molecular formula: (HO)x 2= H 2 O 2
- Slides: 13