EMPIRICAL FORMULA ANSWERS 1 Determine the empirical formula













- Slides: 14

EMPIRICAL FORMULA ANSWERS

• 1. Determine the empirical formula of a compound that contains 2. 94 g oxygen and 1. 96 g sulfur. 1 mol • 2. 94 g oxygen x -------- = 0. 184 mol 16 g 1 mol • 1. 96 g sulfur x --------- = 0. 0613 mol 32 g THIS COLOR - USE THE PERIODIC TABLE

• Divide each by the smallest amount of mol. 0. 0613 S = ----- = 1 0. 0613 0. 184 O = ---- = 3 0. 0613 SO 3

1 mol • 40. 3 g K x -------- = 39 g 1. 03 mol 1 mol • 26. 8 g Cr x --------- = 0. 525 mol 52 g 1 mol • 32. 9 g O x -------- = 16 g 2. 06 mol THIS COLOR - USE THE PERIODIC TABLE • 2. What is the empirical formula of a compound that contains 40. 3% potassium, 26. 8% chromium, and 32. 9% oxygen?

• Divide each by the smallest amount of mol. 1. 03 K = ----- = 2 2. 06 0. 525 O = ---- = 4 0. 525 Cr = ---- = 1 0. 525 2 4 K Cr. O

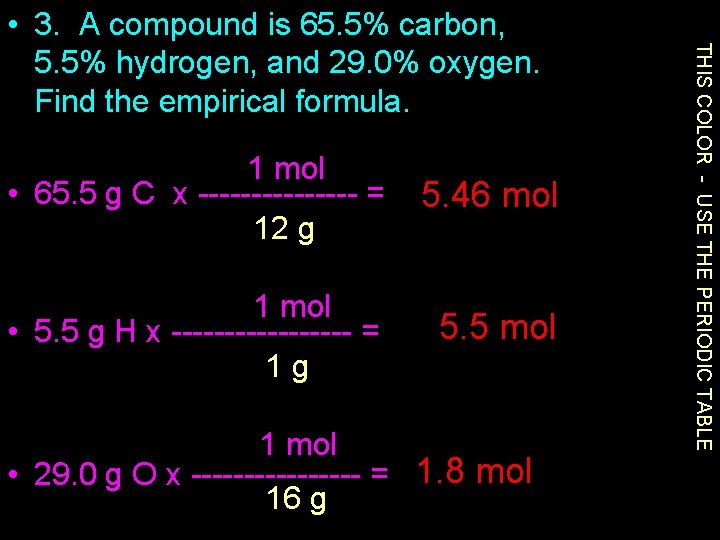
1 mol • 65. 5 g C x -------- = 12 g 5. 46 mol 1 mol • 5. 5 g H x --------- = 1 g 5. 5 mol 1 mol • 29. 0 g O x -------- = 1. 8 mol 16 g THIS COLOR - USE THE PERIODIC TABLE • 3. A compound is 65. 5% carbon, 5. 5% hydrogen, and 29. 0% oxygen. Find the empirical formula.

• Divide each by the smallest amount of mol. 5. 45 C = ----- = 3 1. 8 O = ---- = 1 1. 8 5. 5 H = ---- = 3 1. 8 3 3 CHO

1 mol • 18. 7 g Li x -------- = 7 g 1 mol • 16. 3 g C x --------- = 12 g 2. 67 mol 1. 36 mol 1 mol • 65 g O x -------- = 4. 06 mol 16 g THIS COLOR - USE THE PERIODIC TABLE • 4. What’s the empirical formula of a molecule containing 18. 7% lithium, 16. 3% carbon, and 65% oxygen?

• Divide each by the smallest amount of mol. 2. 67 Li = ----- = 2 4. 06 1. 36 O = ---- = 3 1. 36 C = ---- = 1 1. 36 2 3 Li CO

MOLECULAR FORMULA ANSWERS

• 1) A compound with an empirical formula of C 2 OH 4 and a molar mass of 88 grams per mole. • C = 12 g x 2 = 24 g Molar mass • O = 16 g ------- • H=1 g x 4= 4 g Empirical Formula • +____ mass • 44 g C 4 H 2 O 8 88 g ------ = 2 44 g

• 2) A compound with an empirical formula of C 4 H 4 O and a molar mass of 136 grams per mole. • C = 12 g x 4 = 48 g Molar mass • H=1 g x 4= 4 g ------- • O = 16 g Empirical Formula • +____ mass • 68 g C 8 H 8 O 2 136 g ------ = 2 68 g

• 3) A compound with an empirical formula of CFBr. O and a molar mass of 254. 7 grams per mole. • C = 12 g Molar mass • F = 19 g ------- • Br = 80 g Empirical Formula • O = 16 g mass • +____ • 127 g C 2 F 2 Br 2 O 2 254. 7 g ---- = 2 127 g

• 4) A compound with an empirical formula of C 2 H 8 N and a molar mass of 46 grams per mole. • C = 12 g x 2 = 24 g Molar mass • H=1 g x 8= 8 g ------- • N = 14 g Empirical Formula • +____ mass • 46 g C 2 H 8 N 46 g ------ = 1 46 g