Emitting to the Truth Page 158 Emitting to



























- Slides: 30

Emitting to the Truth Page 158

Emitting to the Truth �Start a new thread/topic �Learning Target: What does color tell us about the underlying structure of matter? �Update TOC

Emitting to the Truth �Read the Introduction p. 158 & 159

Emitting to the Truth �We are going to be looking at different sources of light. �Incandescent light bulb: an electric light which produces light with a filament wire heated to a high temperature by an electric current passing through it, until it glows

Emitting to the Truth �fluorescent light bulb: an low pressure gas filled lamp that uses fluorescence to produce visible light.

Emitting to the Truth �sunlight: a portion of the electromagnetic radiation given off by the Sun, particularly infrared, visible, and ultraviolet light.

Emitting to the Truth �We will be using a spectroscope, which is an instrument that separates light into its constituent wavelengths.

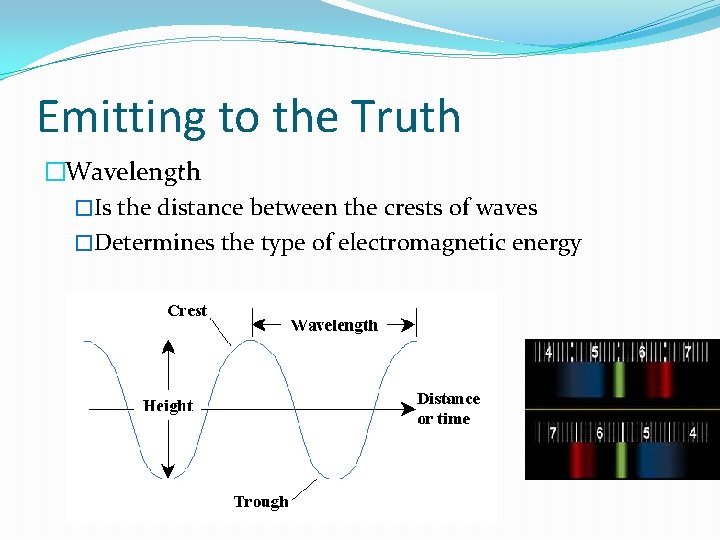
Emitting to the Truth �The spectroscope will show the spectrum of colors that make up the light that is being studied. �spectrum: the range of colors observed when white light is dispersed through a prism �Spectra is the plural of spectrum.

Emitting to the Truth �Each element has a unique spectrum. �Spectra are like fingerprints. �Spectra are characteristic properties of substances and can be used to identify an element.

Emitting to the Truth �Your spectroscope has a scale on it to show the wavelength of each color that makes up the light source. �The lines that appear are called spectral lines.

Emitting to the Truth �Wavelength �Is the distance between the crests of waves �Determines the type of electromagnetic energy

Emitting to the Truth �Electromagnetic spectrum �Visible light is a small portion of the electromagnetic spectrum �The color depends on the wavelength

Emitting to the Truth �Let’s look at the spectra for different elements: Emission Spectra of Elements Beloit College Emission & Absorption Spectra Light 1 Light 2 Light 3 Light 4

Emitting to the Truth �Set up a data table to capture the spectrum for each light source you will be viewing. �Use the spectroscope to view the light source �Use colored pencils to sketch the spectrum in your notebook. �There are 11 lights that Light 1 you are observing. Light 2 �Answer 5 a-e p. 160 Light 3 �Answer R&C #1 -4 �Read/Notes “Energy” p. 161 -162 Light 4

Incandescent Light �Continuous spectrum �All wavelengths are present in the light that is emitted.

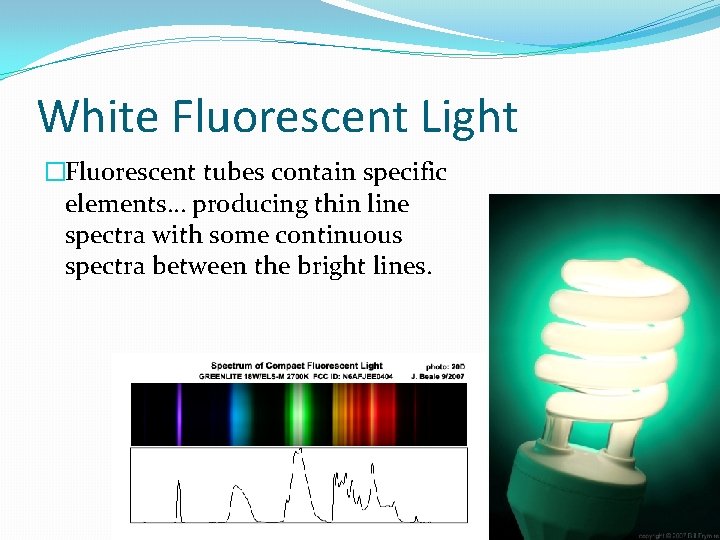
White Fluorescent Light �Fluorescent tubes contain specific elements… producing thin line spectra with some continuous spectra between the bright lines.

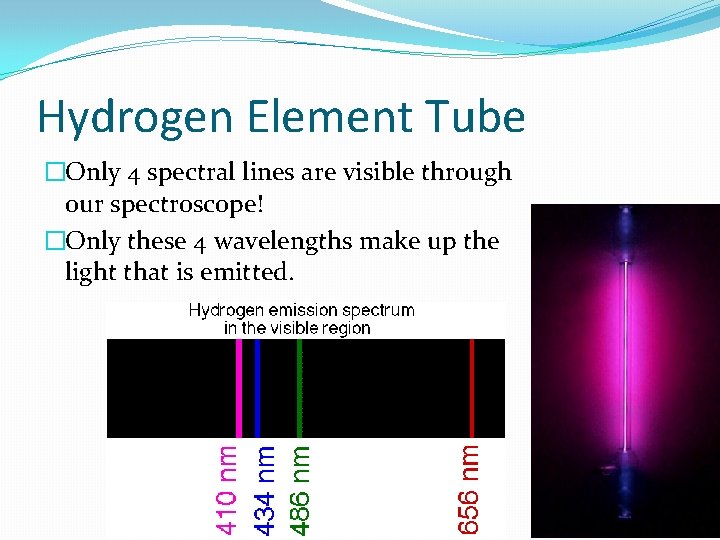
Hydrogen Element Tube �Only 4 spectral lines are visible through our spectroscope! �Only these 4 wavelengths make up the light that is emitted.

Emitting to the Truth �Fluorescent tubes contain specific elements… producing thin line spectra. ��� Incandescent Light bulbs have a thin wire through which electricity runs and burns white-hot… like a campfire, or like the sun! �Remember that each color has a different wavelength, which means a different amount of energy… �� Emission spectra can tell us about the composition of objects… like stars for instance!

Take Home Lessons �In the colored light, blue light has a shorter wavelength = higher frequency = higher energy(compared to red which has long wavelength) �Light is Energy! �We can use a prism (or diffraction grating) to separate light into an emission spectrum. �It is very important to know that some of the light is not visible to humans! It might be infrared, or ultraviolet radiation!

Take Home Lessons �Sometimes, scientists form explanations for things they cannot observe directly. For instance, if you see a broken window and a baseball lying inside, it might be reasonable to infer that the ball did the damage. �It is reasonable to infer that energy is transferred from one ‘thing’ to another… light into heat, or chemical energy into mechanical.

Energy: The Ultimate Quick Change Artist �Energy is the ability to do work �Energy can be measured only by what it does �Energy is abstract - you cannot see it but you can detect it by the changes it effects �Energy can cause changes in temperature, height, velocity, bonds in a molecule, the state of an electron around an atom �Energy is not created or destroyed - it just changes forms �You observe energy changes which lead you to make conclusions about how energy is distributed.

P&P #5 � 5 a. Were all spectra continuous? �Not all spectra were continuous. The incandescent light bulb produced a continuous spectrum, and the fluorescent tube produced a partially continuous spectrum.

P&P #5 � 5 b. Did all spectra produce thin lines of color? �The fluorescent tubes produced thin lines of color.

P&P #5 � 5 c. Did all spectra show bands of black space with no color? �No, the fluorescent tubes had bright lines, but the space between the lines was mostly filled with color, not black lines.

P&P #5 � 5 d. Were thin lines of color always separated by the same amount of black space? �No, the thin lines of color of different elements are separated by different amounts of black space.

P&P #5 � 5 e. Were thin lines of color always located in the same place when comparing 2 different sources? �No, thin lines for the element and fluorescent tubes were in different locations.

R&C #1 �What function does energy serve in the flame tests? What function does energy serve in the different light sources?

R&C #2 �Think about the spectra you might see when viewing light from flame tests. Now remember the spectra from the light sources in this activity. Should spectra from flame tests be similar to any of the 3 light source spectra? If so, which ones and why?

R&C #3 �Think about the spectrum you might see when viewing a white hot campfire. Should the spectrum from a campfire be similar to any of the 3 light source spectra? If so, which one or ones and why?

R&C #4 �Light is a form of energy. Otherwise oceans would not warm and skin would not sunburn. Is every color of light emitted from a light source associated with exactly the same amount of energy? Connect your answer to evidence from flame tests and spectra.
 The verb ser (p. 158) answers
The verb ser (p. 158) answers 158 164
158 164 Scp 158
Scp 158 Krs 158
Krs 158 Cs 158
Cs 158 The verb ser (p. 158) answers
The verb ser (p. 158) answers Construction of light emitting diode
Construction of light emitting diode Vertical cavity surface emitting laser
Vertical cavity surface emitting laser Led light india
Led light india Edge emitting laser
Edge emitting laser Emit and reflect
Emit and reflect An ambulance with a siren emitting a whine at
An ambulance with a siren emitting a whine at Energy band of pn junction
Energy band of pn junction Mla format title page
Mla format title page Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Ví dụ về giọng cùng tên
Ví dụ về giọng cùng tên 101012 bằng
101012 bằng Thể thơ truyền thống
Thể thơ truyền thống Chúa yêu trần thế
Chúa yêu trần thế Hổ đẻ mỗi lứa mấy con
Hổ đẻ mỗi lứa mấy con đại từ thay thế
đại từ thay thế Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Công thức tính độ biến thiên đông lượng
Công thức tính độ biến thiên đông lượng Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Lời thề hippocrates
Lời thề hippocrates Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra độ dài liên kết
độ dài liên kết