Emerging Trends in Safety and Efficacy Venu Menon















- Slides: 22

Emerging Trends in Safety and Efficacy Venu Menon MD, FACC, FAHA Director Cardiac Intensive Care Unit, Director Cardiovascular Fellowship, Associate Director C 5 Professor Of Medicine Cleveland Clinic Lerner College of Medicine

Emerging Trends in Safety and Efficacy

Emerging Trends in Safety and Efficacy It was six men of Indostan to learning much inclined, Who went to see the Elephant (Though all of them were blind), That each by observation…might satisfy his mind. John Saxes (1816 -1887)

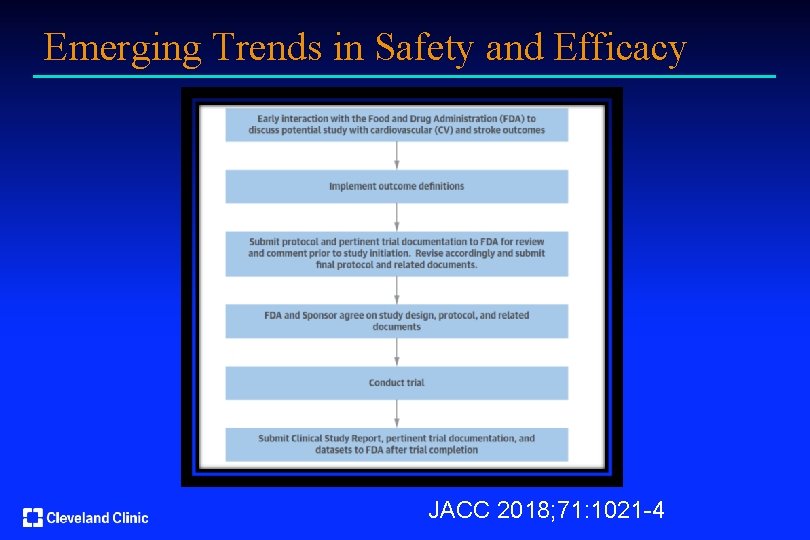
Emerging Trends in Safety and Efficacy JACC 2018; 71: 1021 -4

Expecting the Unexpected:

Expecting the Unexpected: · Cast a broad net.

"Pain is a more terrible lord of mankind than even death itself. " -Albert Schweitzer, MD “Facial Expression of Pain, ” Sir Charles Bell, 1865

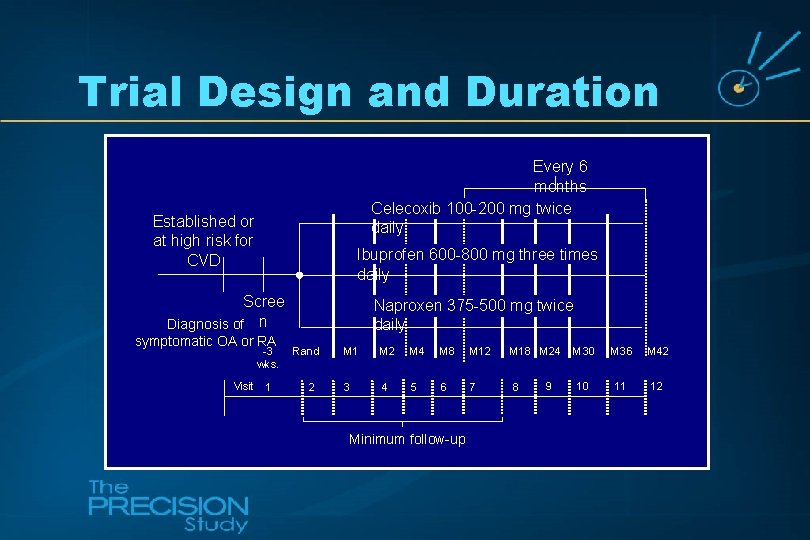
Trial Design and Duration Every 6 months Celecoxib 100 -200 mg twice daily Established or at high risk for CVD Ibuprofen 600 -800 mg three times daily Scree Diagnosis of n symptomatic OA or RA -3 wks. Visit 1 Naproxen 375 -500 mg twice daily Rand 2 M 1 M 2 M 4 M 8 M 12 M 18 M 24 3 4 5 6 7 8 Minimum follow-up 9 M 30 M 36 M 42 10 11 12

Primary Objective To assess the effects of celecoxib 100 -200 mg bid and ibuprofen 600 -800 mg tid compared to naproxen 375 -500 mg bid on the first occurrence of the Antiplatelet Trialists’ Collaboration (APTC) composite cardiovascular endpoint (CV death, non-fatal MI, non-fatal stroke)

Secondary Objectives To compare and evaluate incidence: – MACE - Composite of CV death, non-fatal MI, non-fatal stroke, hospitalization for unstable angina, revascularization, hospitalization for TIA – Clinically significant GI events (CSGIEs) – Effects on renal function and blood pressure – Arthritis efficacy: pain, global improvement, function

Expecting the Unexpected: · Cast a broad net. · Set up Infrastructure.

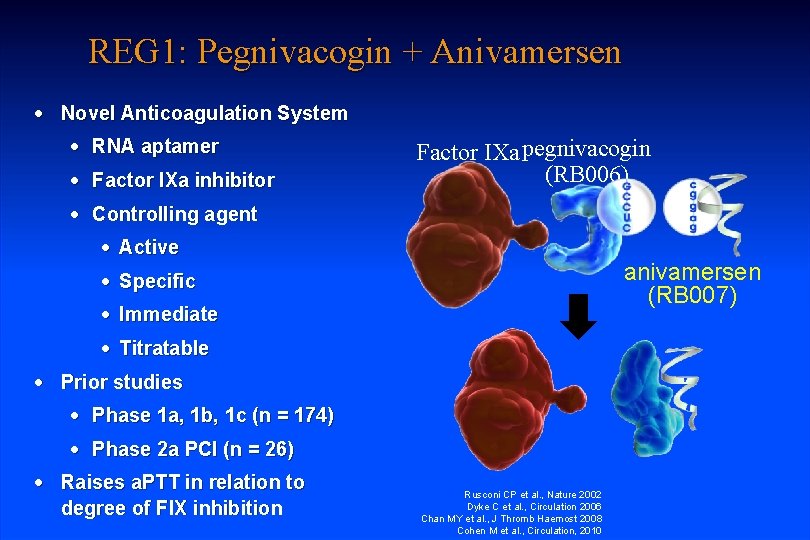
REG 1: Pegnivacogin + Anivamersen · Novel Anticoagulation System · RNA aptamer · Factor IXa inhibitor Factor IXa pegnivacogin (RB 006) · Controlling agent · Active anivamersen (RB 007) · Specific · Immediate · Titratable · Prior studies · Phase 1 a, 1 b, 1 c (n = 174) · Phase 2 a PCI (n = 26) · Raises a. PTT in relation to degree of FIX inhibition Rusconi CP et al. , Nature 2002 Dyke C et al. , Circulation 2006 Chan MY et al. , J Thromb Haemost 2008 Cohen M et al. , Circulation, 2010

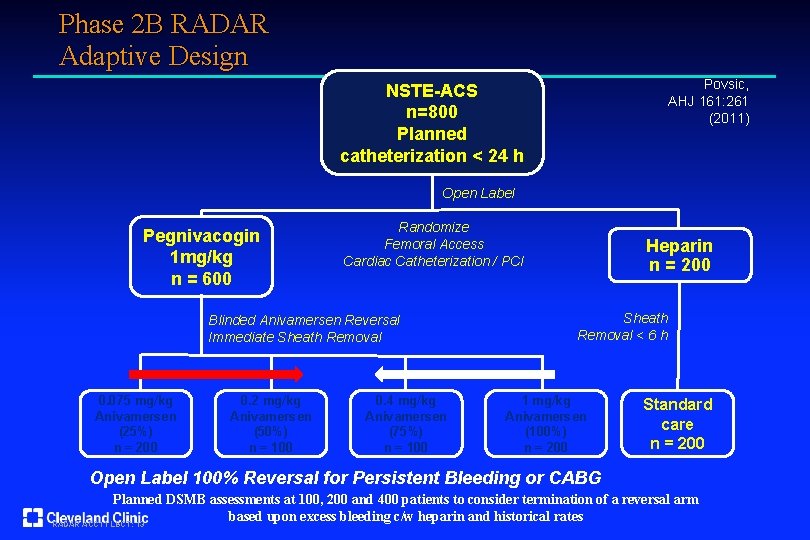
Phase 2 B RADAR Adaptive Design Povsic, AHJ 161: 261 (2011) NSTE-ACS n=800 Planned catheterization < 24 h Open Label Pegnivacogin 1 mg/kg n = 600 Randomize Femoral Access Cardiac Catheterization / PCI Blinded Anivamersen Reversal Immediate Sheath Removal 0. 075 mg/kg Anivamersen (25%) n = 200 0. 2 mg/kg Anivamersen (50%) n = 100 0. 4 mg/kg Anivamersen (75%) n = 100 Heparin n = 200 Sheath Removal < 6 h 1 mg/kg Anivamersen (100%) n = 200 Standard care n = 200 Open Label 100% Reversal for Persistent Bleeding or CABG Planned DSMB assessments at 100, 200 and 400 patients to consider termination of a reversal arm based upon excess bleeding c/w heparin and historical rates RADAR ACC 11 LBCT: 13


Expecting the Unexpected: · Cast a broad net. · Set up Infrastructure. · Consistency of definition and endpoint across a drug class

Normal Kidney Glucose Handling Majority of glucose is reabsorbed by SGLT 2 (90%) Proximal tubule Glucose Filtration ~180 g/day Remaining glucose is reabsorbed by SGLT 1 (10%) Minimal to no glucose excretion Wright EM. Am J Physiol Renal Physiol 2001; 280: F 10– 18; Lee YJ, et al. Kidney Int Suppl 2007; 106: S 27– 35; Hummel CS, et al. Am J Physiol Cell Physiol 2011; 300: C 14– 21

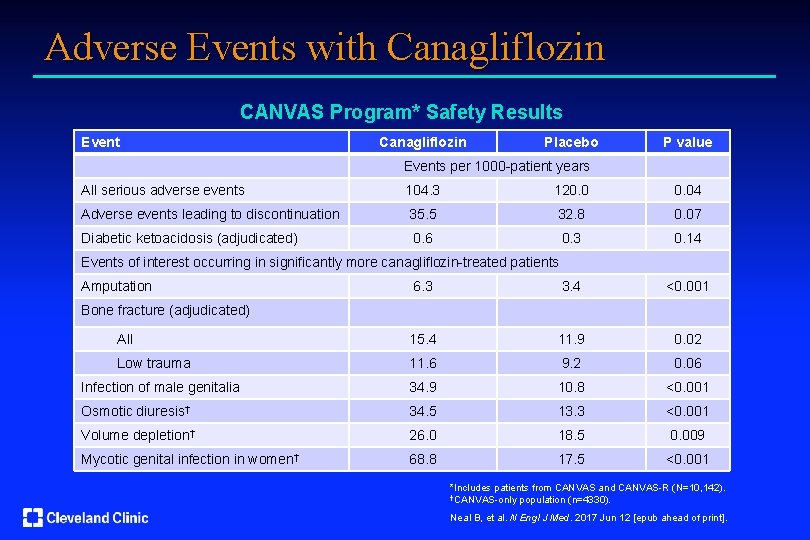
Adverse Events with Canagliflozin CANVAS Program* Safety Results Event Canagliflozin Placebo P value Events per 1000 -patient years All serious adverse events 104. 3 120. 04 Adverse events leading to discontinuation 35. 5 32. 8 0. 07 Diabetic ketoacidosis (adjudicated) 0. 6 0. 3 0. 14 6. 3 3. 4 <0. 001 All 15. 4 11. 9 0. 02 Low trauma 11. 6 9. 2 0. 06 Infection of male genitalia 34. 9 10. 8 <0. 001 Osmotic diuresis† 34. 5 13. 3 <0. 001 Volume depletion† 26. 0 18. 5 0. 009 Mycotic genital infection in women† 68. 8 17. 5 <0. 001 Events of interest occurring in significantly more canagliflozin-treated patients Amputation Bone fracture (adjudicated) *Includes patients from CANVAS and CANVAS-R (N=10, 142). †CANVAS-only population (n=4330). Neal B, et al. N Engl J Med. 2017 Jun 12 [epub ahead of print].

Empagliflozin and Lower Limb Amputation Diabetes Care; 2018; 41: e 4 -5

Canagliflozin and Amputation · Is this a chance finding? · Is this finding specific to canagliflozin? · Is this a class- effect concern for SGLT-2 inhibitors? · Ongoing clinical trials with dapagliflozin and ertugliflozin. Researchers observed no evidence of increased risk of below-theknee amputation with canagliflozin vs. dapagliflozin and empagliflozin in both the overall population (HR = 1. 14; 95% CI, 0. 67 -1. 93) and among patients with established CVD (HR = 1. 08; 95% CI, 0. 63 -1. 82). Similar results were observed when comparing canagliflozin with all non-SGLT 2 therapies, with no increased risk in the overall type 2 diabetes population (HR = 0. 75; 95% CI, 0. 4 -1. 41) or among those with established CVD (HR = 0. 72’ 95% CI, 0. 341. 51), JAMA Intern Med 2018; 178: 1190 -98 OBSERVE 4 D

Expecting the Unexpected: · Cast a broad net. · Set up Infrastructure. · Consistency of definition and endpoint across a drug class · Concurrent and Electronic Adjudication

Advantages of the Electronic Record · Accurate and concurrent review of events. · Transparency in workflow status. · Immediate data accessibility via analytics. · Integration with EDC systems to minimize data entry.

Expecting the Unexpected: · Cast a broad net. · Set up Infrastructure. · Consistency of definition and endpoint across a drug class · Concurrent and Electronic Adjudication · Quality Control
 Divya menon md
Divya menon md Trends in community development
Trends in community development Emerging trends in business intelligence
Emerging trends in business intelligence Emerging trends in software engineering ppt
Emerging trends in software engineering ppt Emerging trends in business intelligence implementation
Emerging trends in business intelligence implementation Emerging trends in strategic management
Emerging trends in strategic management Emerging trends in society
Emerging trends in society Challenges of emerging trends in operating systems
Challenges of emerging trends in operating systems Emerging trends in disaster management
Emerging trends in disaster management Mis trends
Mis trends Je suis venu pour la vie
Je suis venu pour la vie Que ma vie soit une fleur
Que ma vie soit une fleur Teachable clone
Teachable clone Dr. m. venu gopala rao
Dr. m. venu gopala rao Dr. m. venu gopala rao
Dr. m. venu gopala rao Jsus
Jsus Gautam menon imsc
Gautam menon imsc Bourgeoisie definition
Bourgeoisie definition Dr divya menon
Dr divya menon Dr geetha menon
Dr geetha menon Nurturing leadership qualities
Nurturing leadership qualities Charcot's triad
Charcot's triad Memory management
Memory management