ELSI Challenges Associated with Clinical Applications of Exome








- Slides: 20

ELSI Challenges Associated with Clinical Applications of Exome Sequencing: preliminary results from a Systematic Literature Review Gabrielle Bertier, Ph. D candidate Mc. Gill University, Toulouse III University Translation in healthcare conference Exploring the impact of emerging technologies University of oxford 23 -25 June 2015

Outline 1. Introduction A. Context & Study objectives B. Methodology 2. Preliminary findings A. Articles overview B. Technology users identified implementation issues 3. Discussion A. Study limitations B. Future steps

1. Introduction A. Context & Objective Ph. D project • Empirical evidence, implementation of whole exome sequencing in the clinical setting • Case studies France/Canada • Data trajectory: from raw sequencing data to clinical information

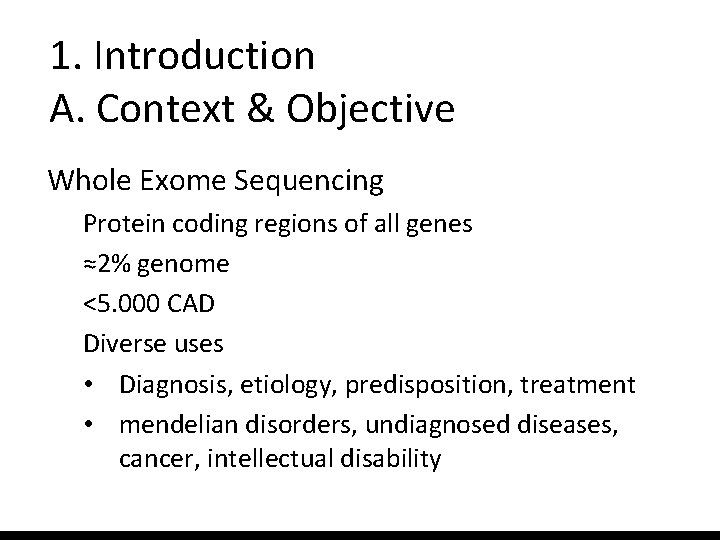
1. Introduction A. Context & Objective Whole Exome Sequencing Protein coding regions of all genes ≈2% genome <5. 000 CAD Diverse uses • Diagnosis, etiology, predisposition, treatment • mendelian disorders, undiagnosed diseases, cancer, intellectual disability

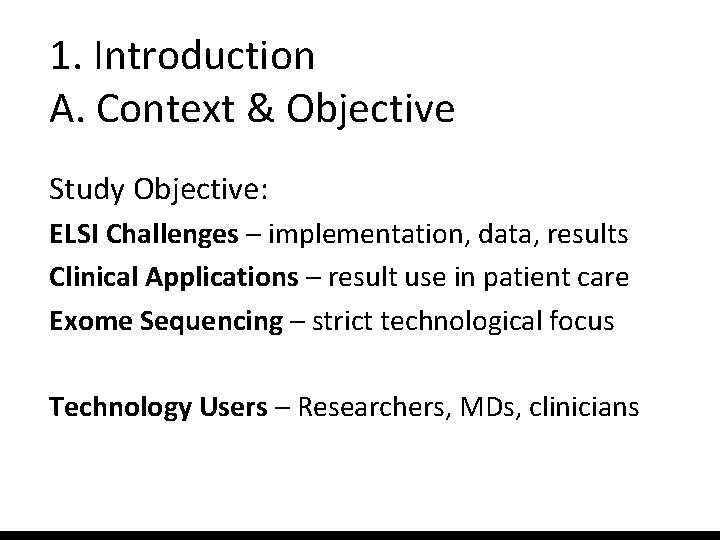
1. Introduction A. Context & Objective Study Objective: ELSI Challenges – implementation, data, results Clinical Applications – result use in patient care Exome Sequencing – strict technological focus Technology Users – Researchers, MDs, clinicians

1. Introduction B. Methodology Search EBSCO, EMBASE, Pub. Med, Science Direct, Scopus, Web of Science (“exome sequencing” OR “whole-exome sequencing” OR “whole exome sequencing”) AND (“Clinical application” OR “Medical application” OR “Healthcare” OR “Clinical care” OR “Medical care” OR “Clinical practice” OR “Clinical diagnostic” OR “Medical practice”)

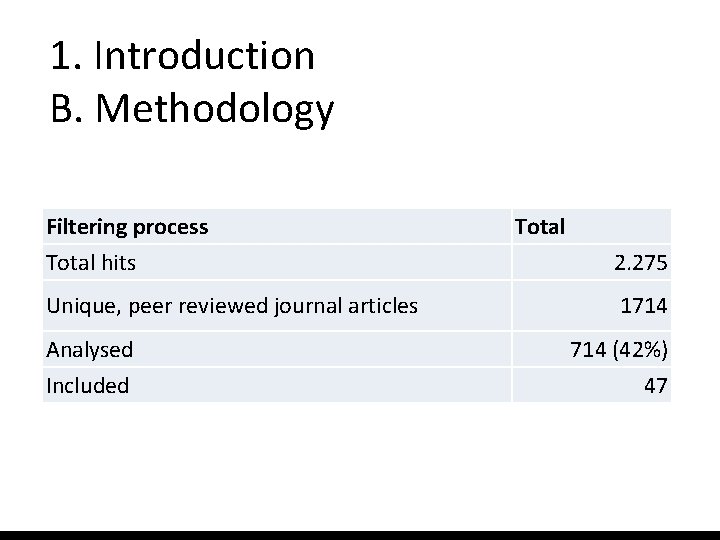
1. Introduction B. Methodology Filtering process Total hits Unique, peer reviewed journal articles Analysed Included Total 2. 275 1714 (42%) 47

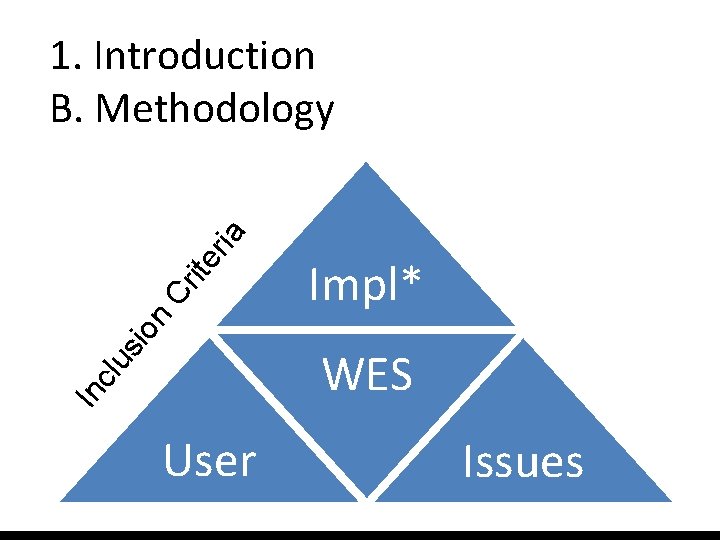
Impl* sio n Cr ite ria 1. Introduction B. Methodology In clu WES User Issues

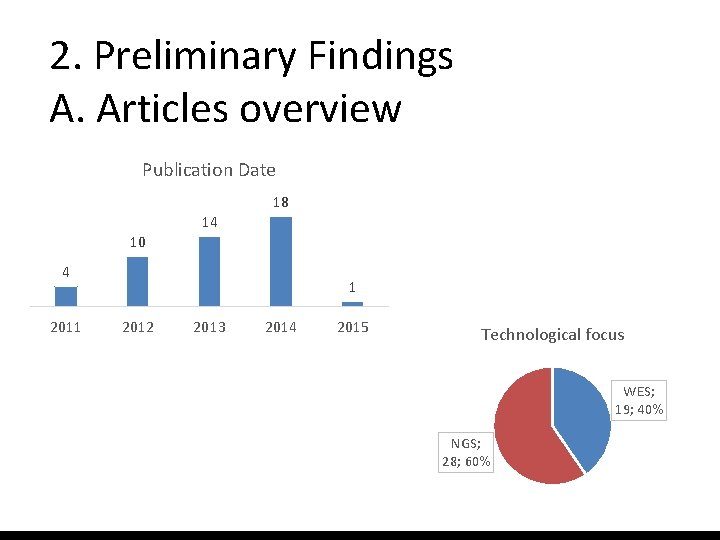
2. Preliminary Findings A. Articles overview Publication Date 18 14 10 4 2011 1 2012 2013 2014 2015 Technological focus WES; 19; 40% NGS; 28; 60%

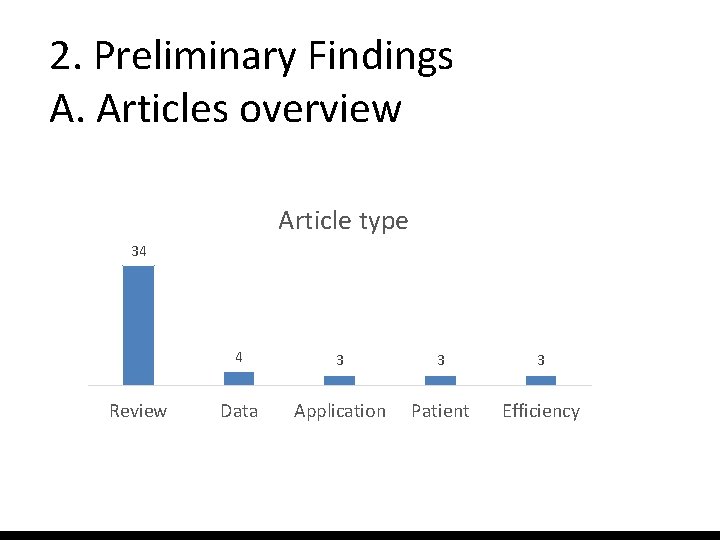
2. Preliminary Findings A. Articles overview Article type 34 Review 4 3 3 3 Data Application Patient Efficiency

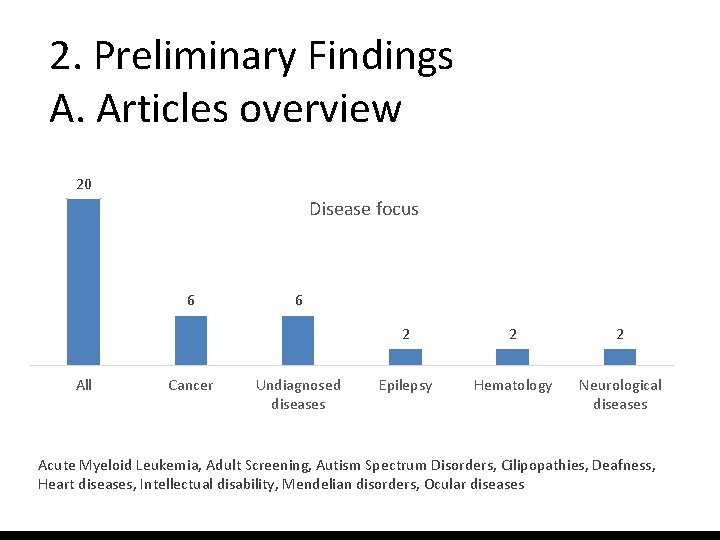
2. Preliminary Findings A. Articles overview 20 Disease focus 6 All Cancer 6 Undiagnosed diseases 2 2 2 Epilepsy Hematology Neurological diseases Acute Myeloid Leukemia, Adult Screening, Autism Spectrum Disorders, Cilipopathies, Deafness, Heart diseases, Intellectual disability, Mendelian disorders, Ocular diseases

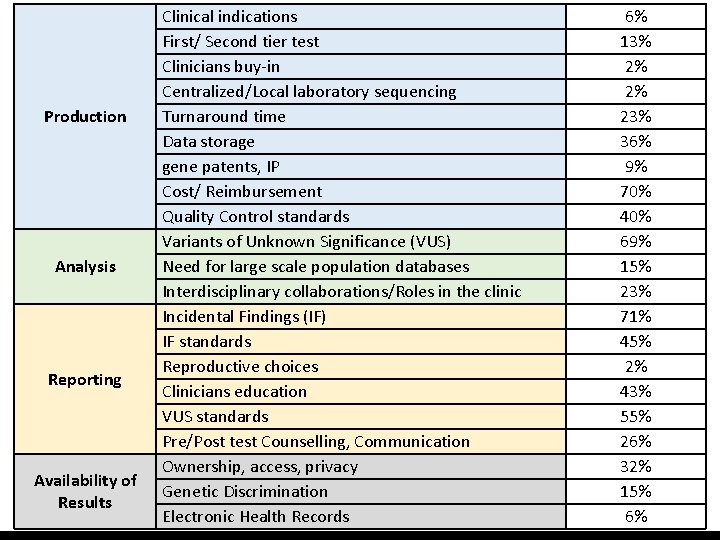
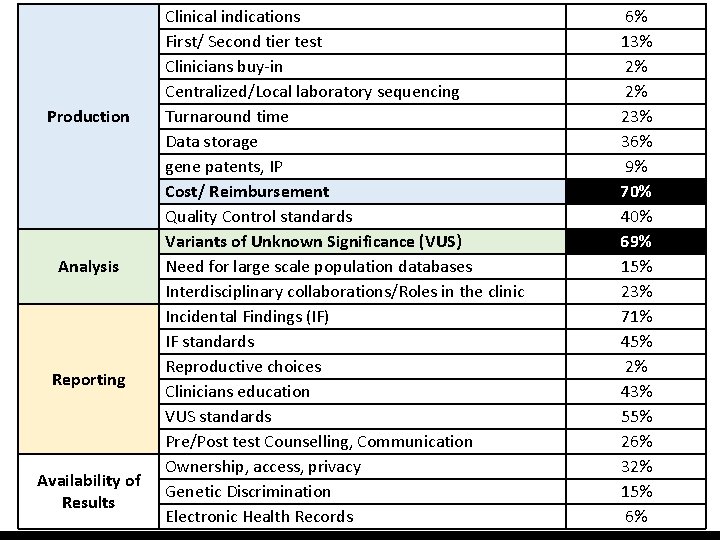
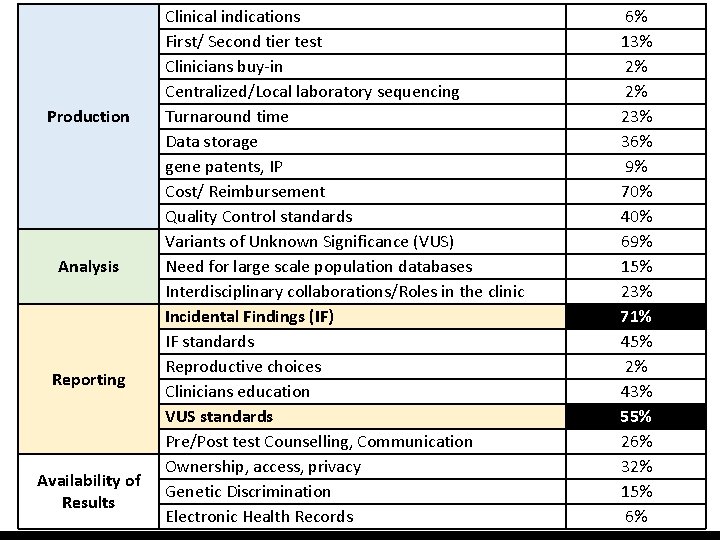
Production Analysis Reporting Availability of Results Clinical indications First/ Second tier test Clinicians buy-in Centralized/Local laboratory sequencing Turnaround time Data storage gene patents, IP Cost/ Reimbursement Quality Control standards Variants of Unknown Significance (VUS) Need for large scale population databases Interdisciplinary collaborations/Roles in the clinic Incidental Findings (IF) IF standards Reproductive choices Clinicians education VUS standards Pre/Post test Counselling, Communication Ownership, access, privacy Genetic Discrimination Electronic Health Records 6% 13% 2% 2% 23% 36% 9% 70% 40% 69% 15% 23% 71% 45% 2% 43% 55% 26% 32% 15% 6%

Production Analysis Reporting Availability of Results Clinical indications First/ Second tier test Clinicians buy-in Centralized/Local laboratory sequencing Turnaround time Data storage gene patents, IP Cost/ Reimbursement Quality Control standards Variants of Unknown Significance (VUS) Need for large scale population databases Interdisciplinary collaborations/Roles in the clinic Incidental Findings (IF) IF standards Reproductive choices Clinicians education VUS standards Pre/Post test Counselling, Communication Ownership, access, privacy Genetic Discrimination Electronic Health Records 6% 13% 2% 2% 23% 36% 9% 70% 40% 69% 15% 23% 71% 45% 2% 43% 55% 26% 32% 15% 6%

Production Analysis Reporting Availability of Results Clinical indications First/ Second tier test Clinicians buy-in Centralized/Local laboratory sequencing Turnaround time Data storage gene patents, IP Cost/ Reimbursement Quality Control standards Variants of Unknown Significance (VUS) Need for large scale population databases Interdisciplinary collaborations/Roles in the clinic Incidental Findings (IF) IF standards Reproductive choices Clinicians education VUS standards Pre/Post test Counselling, Communication Ownership, access, privacy Genetic Discrimination Electronic Health Records 6% 13% 2% 2% 23% 36% 9% 70% 40% 69% 15% 23% 71% 45% 2% 43% 55% 26% 32% 15% 6%

2. Preliminary Findings B. Implementation Issues • Implementation underway: technological push • Wide range of issues • Clear call for Standards

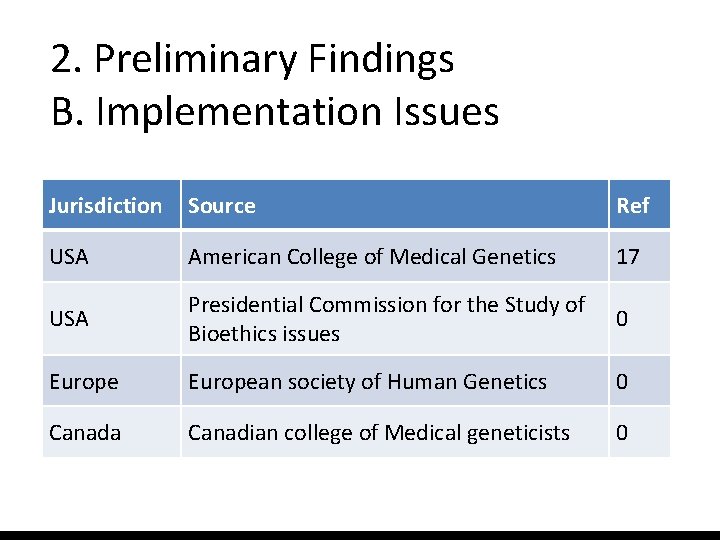
2. Preliminary Findings B. Implementation Issues Who should regulate? • Professional societies, disease specific implementation regulation? • Hospital policies? (IF, VUS) • Regional/national policies (QC? ) • Others

2. Preliminary Findings B. Implementation Issues Jurisdiction Source Ref USA American College of Medical Genetics 17 USA Presidential Commission for the Study of Bioethics issues 0 European society of Human Genetics 0 Canada Canadian college of Medical geneticists 0

3. Discussion A. Study limitations • Search, filtering and inclusion criteria • Preliminary Results (42% results analysed only) • Analysis structure and focus

3. Discussion B. Future Steps • Second researcher looking through the data • Finish data analysis • Analysis of current regulatory framework

Acknowledgements Professor Yann Joly Professor Bartha Maria Knoppers Martin Hétu Professor Anne Cambon Thomsen
 Exome sequencing project
Exome sequencing project Exome
Exome Chirocredits
Chirocredits Foundations of clinical research applications to practice
Foundations of clinical research applications to practice Adeno associated virus biosafety level
Adeno associated virus biosafety level Stigma associated with failure and repetition is removed
Stigma associated with failure and repetition is removed Berstoff gearbox repair
Berstoff gearbox repair Chronic hyperplastic candidiasis
Chronic hyperplastic candidiasis Arithmetic population density
Arithmetic population density Ivan pavlov is most closely associated with
Ivan pavlov is most closely associated with Utah associated municipal power systems
Utah associated municipal power systems Symbol for associated with
Symbol for associated with Colors associated with time
Colors associated with time Knightley v johns
Knightley v johns Big data analytics is usually associated with
Big data analytics is usually associated with Psychodynamic theory is most closely associated with
Psychodynamic theory is most closely associated with What encompasses all activities associated with the flow
What encompasses all activities associated with the flow Problems of water resources
Problems of water resources Everett hagen theory of entrepreneurship
Everett hagen theory of entrepreneurship Post office vocabulary words
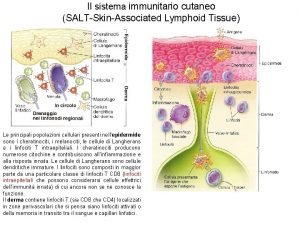
Post office vocabulary words Salt skin associated lymphoid tissue
Salt skin associated lymphoid tissue