Elixir Medical Corporation Biodegradable Polymer Program Motasim Sirhan

Elixir Medical Corporation Biodegradable Polymer Program Motasim Sirhan, CEO Elixir Medical DESolve TM : Investigational device, not available for sale in the US DESyne ® and DESyne BD ® : CE mark approved, not available for sale in the US
Elixir Medical: Innovating Vascular Restoration Developing drug-device therapies to improve the standard of care for vascular intervention § Bilingual organization fluent in the development of drugs and devices for vascular applications § Developed and patented proprietary drug, Novolimus, a metabolite of Sirolimus with high potency and known safety profile § Developed highly biocompatible, biodegradable polymeric materials designed to sustain excellent long term clinical outcomes Industry’s most comprehensive portfolio of drug eluting coronary vascular therapies § DESolve. TM fully bioresorbable scaffold: enrolled pivotal trial for CE Mark, approval anticipated in 2013 § DESyne BD® biodegradable polymer DES: CE Mark approved, launching in 2013 § DESyne® durable polymer DES: CE Mark approved, beginning commercialization Leverage proprietary know-how and technologies for peripheral vascular therapies § Adapting our bioresorbable scaffolds to SFA, BTK and pediatric applications where the need for improved therapies is acute and bringing them into the clinic Elixir Medical 2
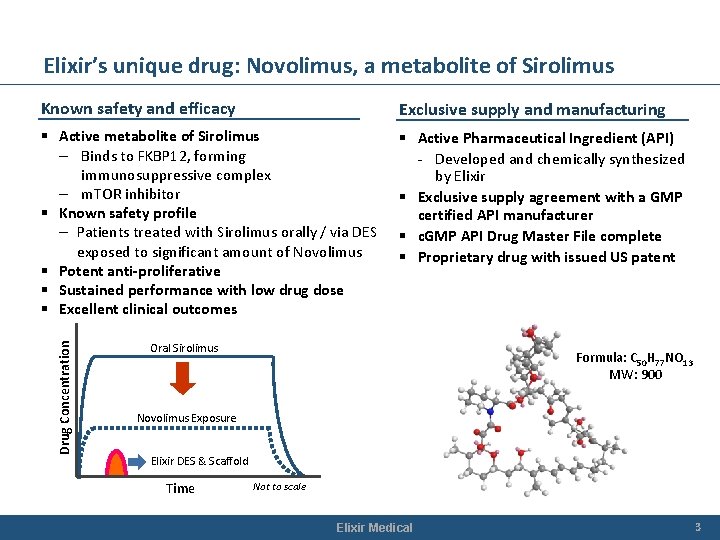
Elixir’s unique drug: Novolimus, a metabolite of Sirolimus Exclusive supply and manufacturing § Active metabolite of Sirolimus – Binds to FKBP 12, forming immunosuppressive complex – m. TOR inhibitor § Known safety profile – Patients treated with Sirolimus orally / via DES exposed to significant amount of Novolimus § Potent anti-proliferative § Sustained performance with low drug dose § Excellent clinical outcomes § Active Pharmaceutical Ingredient (API) - Developed and chemically synthesized by Elixir § Exclusive supply agreement with a GMP certified API manufacturer § c. GMP API Drug Master File complete § Proprietary drug with issued US patent Drug Concentration Known safety and efficacy Oral Sirolimus Formula: C 50 H 77 NO 13 MW: 900 Novolimus Exposure Elixir DES & Scaffold Time Not to scale Elixir Medical 3
Elixir has the broadest DES product portfolio in the industry for the markets of today and tomorrow DESolve Breakthrough bioresorbable scaffold DESyne BD Biodegradable polymer DES designed to reduce the duration of DAPT DESyne Workhorse DES with best-in-class safety, efficacy Elixir Medical 4
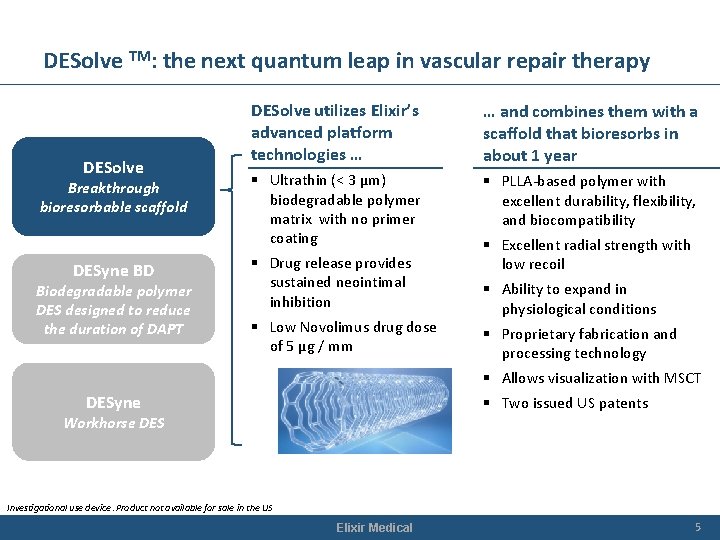
DESolve TM: the next quantum leap in vascular repair therapy DESolve Breakthrough bioresorbable scaffold DESyne BD Biodegradable polymer DES designed to reduce the duration of DAPT DESolve utilizes Elixir’s advanced platform technologies … … and combines them with a scaffold that bioresorbs in about 1 year § Ultrathin (< 3 µm) biodegradable polymer matrix with no primer coating § PLLA-based polymer with excellent durability, flexibility, and biocompatibility § Drug release provides sustained neointimal inhibition § Low Novolimus drug dose of 5 µg / mm § Excellent radial strength with low recoil § Ability to expand in physiological conditions § Proprietary fabrication and processing technology § Allows visualization with MSCT DESyne § Two issued US patents Workhorse DES Investigational use device. Product not available for sale in the US Elixir Medical 5
DESolve. TM has several key differentiating performance characteristics 1 Scaffold bioresorbs in about 1 year and provides excellent low late lumen loss at 6 months 2 Self-correcting scaffold property designed to resolve malapposition 3 Substantial safety margin for fracture resistance 4 Wide range of sizes to accommodate a broad range of vessel sizes and lesion lengths Achieving ease of use and performance characteristics comparable with metallic DES systems Elixir Medical 6

DESolve. TM has demonstrated success in the clinic First-In-Man trial complete with excellent 6 month and 12 month results § 16 patients across Belgium and New Zealand § Primary endpoint: in-stent late lumen loss at 6 months – 0. 19 mm late lumen loss § Principal investigators: Dr. Stefan Verheye and Dr. John Ormiston DESolve Nx CE Mark pivotal trial undergoing follow-up § 126 patients across 15 sites: Europe, New Zealand, and Brazil § Principal investigators: Dr. Alexandre Abizaid, Dr. Joachim Schofer, Dr. Stefan Verheye § Primary endpoint: in-stent late lumen loss at 6 months; other endpoints: – Clinical: MACE, stent thrombosis – QCA: In-segment late lumen loss, binary restenosis, and percent diameter stenosis – Subset of patients: QCA, IVUS, OCT at 6, 12 and 24 months; MSCT at 12 months § Data analysis ongoing – 6 month clinical and imaging data to be presented at Euro PCR 2013 § CE Mark approval anticipated in 2013 Elixir Medical 7
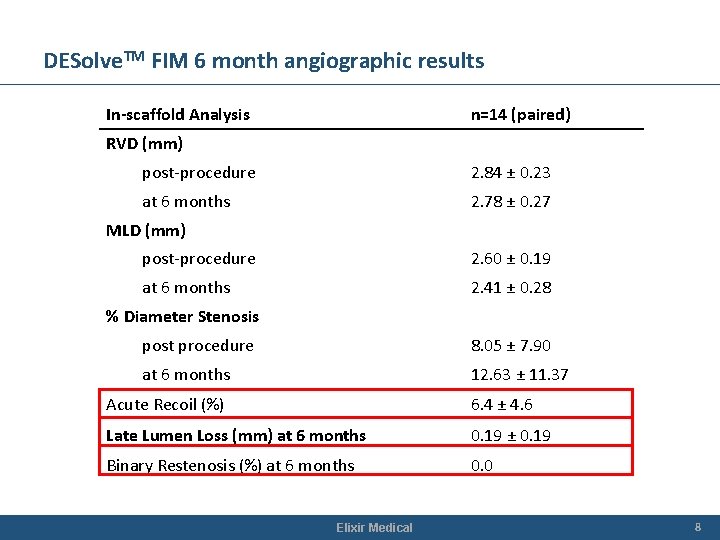
DESolve. TM FIM 6 month angiographic results In-scaffold Analysis n=14 (paired) RVD (mm) post-procedure 2. 84 ± 0. 23 at 6 months 2. 78 ± 0. 27 MLD (mm) post-procedure 2. 60 ± 0. 19 at 6 months 2. 41 ± 0. 28 % Diameter Stenosis post procedure 8. 05 ± 7. 90 at 6 months 12. 63 ± 11. 37 Acute Recoil (%) 6. 4 ± 4. 6 Late Lumen Loss (mm) at 6 months 0. 19 ± 0. 19 Binary Restenosis (%) at 6 months 0. 0 Elixir Medical 8
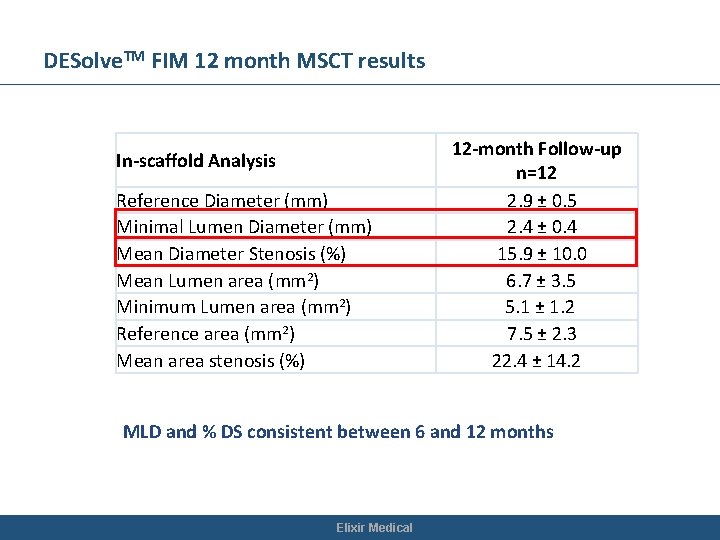
DESolve. TM FIM 12 month MSCT results In-scaffold Analysis Reference Diameter (mm) Minimal Lumen Diameter (mm) Mean Diameter Stenosis (%) Mean Lumen area (mm 2) Minimum Lumen area (mm 2) Reference area (mm 2) Mean area stenosis (%) 12 -month Follow-up n=12 2. 9 ± 0. 5 2. 4 ± 0. 4 15. 9 ± 10. 0 6. 7 ± 3. 5 5. 1 ± 1. 2 7. 5 ± 2. 3 22. 4 ± 14. 2 MLD and % DS consistent between 6 and 12 months Elixir Medical
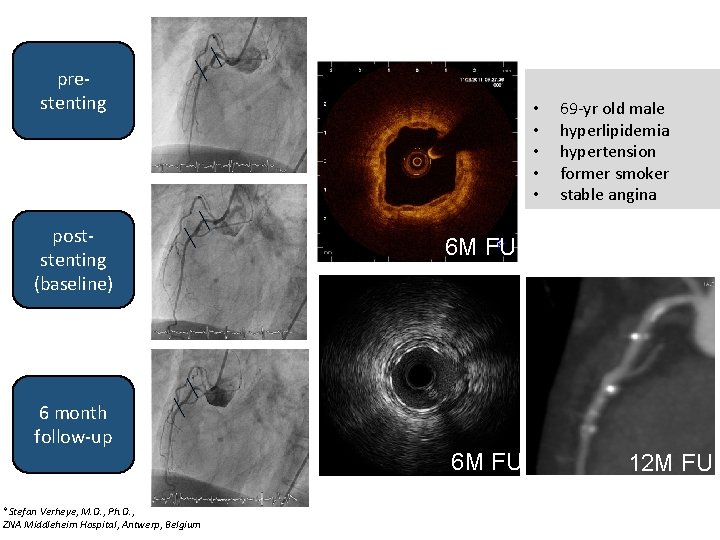
prestenting poststenting (baseline) • • • 69 -yr old male hyperlipidemia hypertension former smoker stable angina 6 M FU 6 month follow-up 6 M FU *Stefan Verheye, M. D. , Ph. D. , ZNA Middleheim Hospital, Antwerp, Belgium 12 M FU
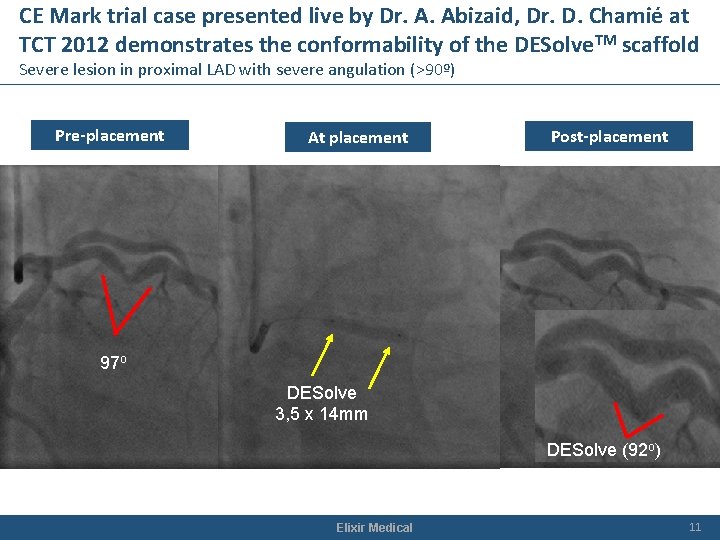
CE Mark trial case presented live by Dr. A. Abizaid, Dr. D. Chamié at TCT 2012 demonstrates the conformability of the DESolve. TM scaffold Severe lesion in proximal LAD with severe angulation (>90º) Pre-placement At placement Post-placement 97 o DESolve 3, 5 x 14 mm DESolve (92 o) Elixir Medical 11
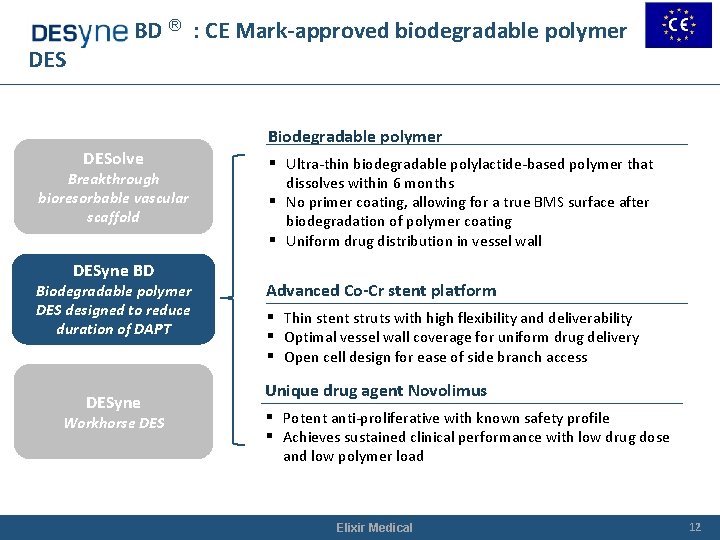
DESyn BD ® : CE Mark-approved biodegradable polymer DESolve Breakthrough bioresorbable vascular scaffold DESyne BD Biodegradable polymer DES designed to reduce duration of DAPT DESyne Workhorse DES Biodegradable polymer § Ultra-thin biodegradable polylactide-based polymer that dissolves within 6 months § No primer coating, allowing for a true BMS surface after biodegradation of polymer coating § Uniform drug distribution in vessel wall Advanced Co-Cr stent platform § Thin stent struts with high flexibility and deliverability § Optimal vessel wall coverage for uniform drug delivery § Open cell design for ease of side branch access Unique drug agent Novolimus § Potent anti-proliferative with known safety profile § Achieves sustained clinical performance with low drug dose and low polymer load Elixir Medical 12
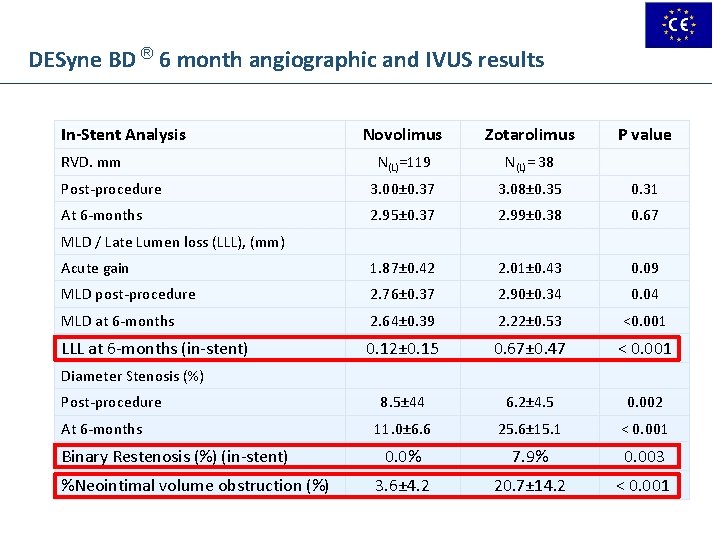
DESyne BD ® 6 month angiographic and IVUS results In-Stent Analysis Novolimus Zotarolimus N(L)=119 N(L)= 38 Post-procedure 3. 00± 0. 37 3. 08± 0. 35 0. 31 At 6 -months 2. 95± 0. 37 2. 99± 0. 38 0. 67 Acute gain 1. 87± 0. 42 2. 01± 0. 43 0. 09 MLD post-procedure 2. 76± 0. 37 2. 90± 0. 34 0. 04 MLD at 6 -months 2. 64± 0. 39 2. 22± 0. 53 <0. 001 LLL at 6 -months (in-stent) 0. 12± 0. 15 0. 67± 0. 47 < 0. 001 8. 5± 44 6. 2± 4. 5 0. 002 11. 0± 6. 6 25. 6± 15. 1 < 0. 001 0. 0% 7. 9% 0. 003 3. 6± 4. 2 20. 7± 14. 2 < 0. 001 RVD. mm P value MLD / Late Lumen loss (LLL), (mm) Diameter Stenosis (%) Post-procedure At 6 -months Binary Restenosis (%) (in-stent) %Neointimal volume obstruction (%)
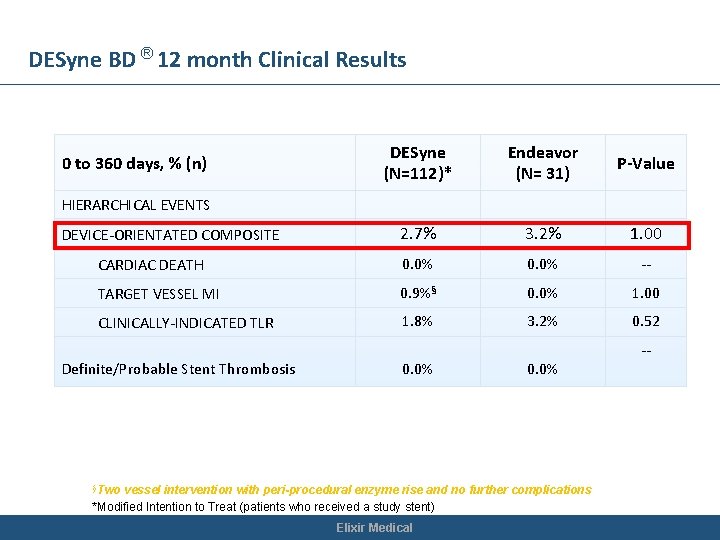
DESyne BD ® 12 month Clinical Results DESyne (N=112)* Endeavor (N= 31) P-Value 2. 7% 3. 2% 1. 00 CARDIAC DEATH 0. 0% -- TARGET VESSEL MI 0. 9%§ 0. 0% 1. 00 CLINICALLY-INDICATED TLR 1. 8% 3. 2% 0. 52 0 to 360 days, % (n) HIERARCHICAL EVENTS DEVICE-ORIENTATED COMPOSITE Definite/Probable Stent Thrombosis 0. 0% §Two 0. 0% vessel intervention with peri-procedural enzyme rise and no further complications *Modified Intention to Treat (patients who received a study stent) Elixir Medical --
CE Mark-approved workhorse DES FORMULA DESolve Breakthrough bioresorbable vascular scaffold DESyne BD Biodegradable polymer DES designed to reduce duration of DAPT DESyne Workhorse DES coating technology § Thin polymer and drug matrix coating without the need for a primer coating § Lowest polymer load for improved biocompatibility Advanced Co-Cr stent platform § Thin stent struts with high flexibility and deliverability § Optimal vessel wall coverage for uniform drug delivery § Open cell design for ease of side branch access Unique drug agent Novolimus § Potent anti-proliferative with known safety profile § Achieves sustained clinical performance with the lowest drug dose among DES on the market Elixir Medical 15
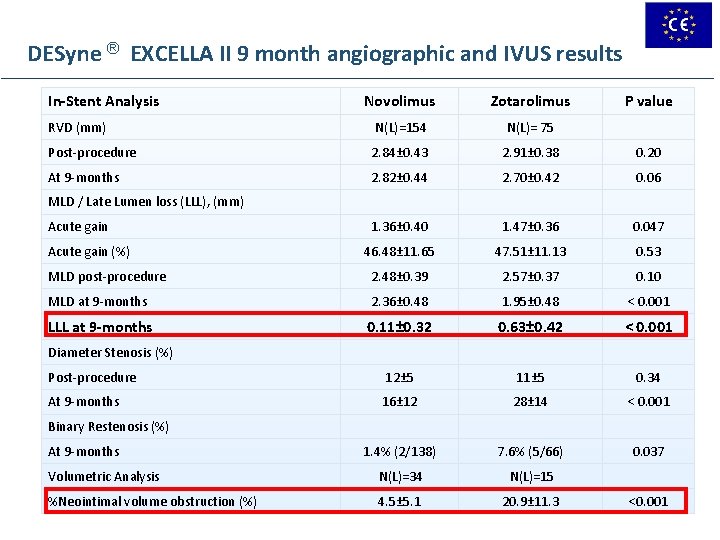
DESyne ® EXCELLA II 9 month angiographic and IVUS results In-Stent Analysis Novolimus Zotarolimus P value RVD (mm) N(L)=154 N(L)= 75 Post-procedure 2. 84± 0. 43 2. 91± 0. 38 0. 20 At 9 -months 2. 82± 0. 44 2. 70± 0. 42 0. 06 1. 36± 0. 40 1. 47± 0. 36 0. 047 46. 48± 11. 65 47. 51± 11. 13 0. 53 MLD post-procedure 2. 48± 0. 39 2. 57± 0. 37 0. 10 MLD at 9 -months 2. 36± 0. 48 1. 95± 0. 48 < 0. 001 LLL at 9 -months 0. 11± 0. 32 0. 63± 0. 42 < 0. 001 Post-procedure 12± 5 11± 5 0. 34 At 9 -months 16± 12 28± 14 < 0. 001 1. 4% (2/138) 7. 6% (5/66) 0. 037 Volumetric Analysis N(L)=34 N(L)=15 %Neointimal volume obstruction (%) 4. 5± 5. 1 20. 9± 11. 3 MLD / Late Lumen loss (LLL), (mm) Acute gain (%) Diameter Stenosis (%) Binary Restenosis (%) At 9 -months <0. 001
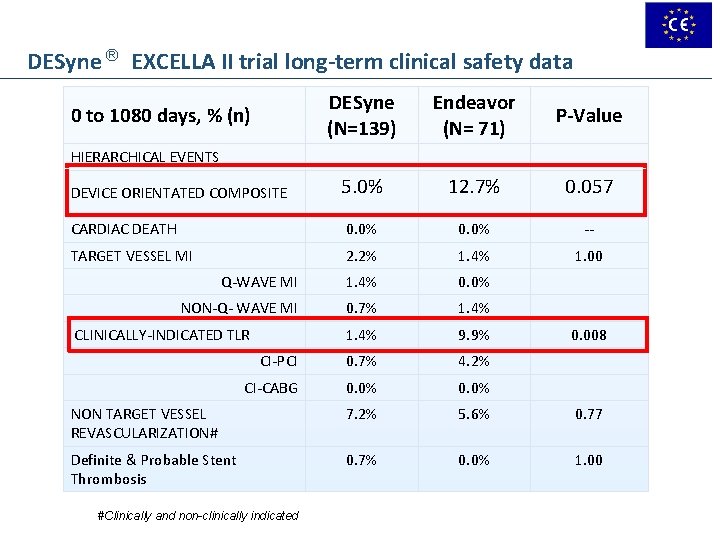
DESyne ® EXCELLA II trial long-term clinical safety data DESyne (N=139) Endeavor (N= 71) P-Value 5. 0% 12. 7% 0. 057 CARDIAC DEATH 0. 0% -- TARGET VESSEL MI 2. 2% 1. 4% 1. 00 Q-WAVE MI 1. 4% 0. 0% NON-Q- WAVE MI 0. 7% 1. 4% 9. 9% CI-PCI 0. 7% 4. 2% CI-CABG 0. 0% NON TARGET VESSEL REVASCULARIZATION# 7. 2% 5. 6% 0. 77 Definite & Probable Stent Thrombosis 0. 7% 0. 0% 1. 00 0 to 1080 days, % (n) HIERARCHICAL EVENTS DEVICE ORIENTATED COMPOSITE CLINICALLY-INDICATED TLR #Clinically and non-clinically indicated 0. 008
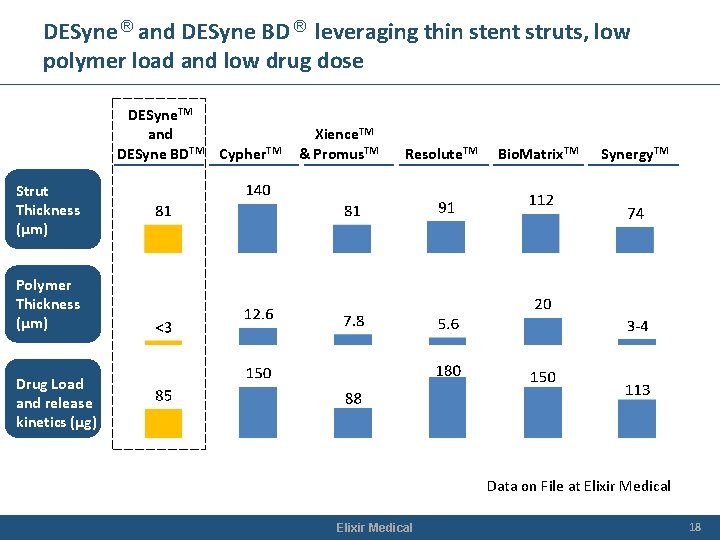
DESyne ® and DESyne BD ® leveraging thin stent struts, low polymer load and low drug dose DESyne. TM and DESyne BDTM Cypher. TM Xience. TM & Promus. TM Resolute. TM Bio. Matrix. TM Synergy. TM Strut Thickness (µm) Polymer Thickness (µm) Drug Load and release kinetics (µg) Data on File at Elixir Medical 18
Elixir is leveraging its proprietary know-how and technologies for peripheral vascular therapies Bioresorbable technologies can improve outcomes, expanding the global peripheral vascular device market Superficial femoral artery Below the knee § Long lesion lengths and complex biomechanics § CLI below the knee is a very severe condition lead to § Suboptimal long term patency rates with POBA and metallic BMS/DES § DCB leaves behind no permanent implant, but with sub-optimal long term patency with ~50% of cases resulting in death or amputation § Sub-optimal patency with POBA, DCB and BMS/DES • DESolve technology can overcome the limitations by offering the required radial support and leaving behind no permanent implant and potentially achieving optimal long term patency Elixir activity • Entered pre-clinical testing in 2012, including pediatric indication • FIM studies in 2013 Elixir activity • Preclinical studies in 2013 Future DESolve application includes iliac, pudendal, renal, carotid and neural arteries… Elixir Medical 19

Elixir technology in new clinical indications Pediatric Applications § Congenital Heart Disease § Pulmonary Artery Stenosis § 1 -2000 births § Coarctation of Aorta § 1 -10, 000 births § Currently treated by surgery/PTI with stenting § Typically requires multiple re-intervention to re-expand stent/surgery to accommodate somatic growth • DESolve technology can overcome the limitations by offering the required radial support , scaffold growth with somatic growth, followed by bioresorption and there by no repeat intervention Elixir activity • Entered pre-clinical testing in 2012 Elixir Medical 20
- Slides: 20