Elimination Rxn Predict the reaction pathway main products





- Slides: 70

Elimination Rxn • Predict the reaction pathway (main products) for E 2 and E 1 • Draw reaction mechanism for E 1 • Design synthetic pathway based on mechanism 7 -1

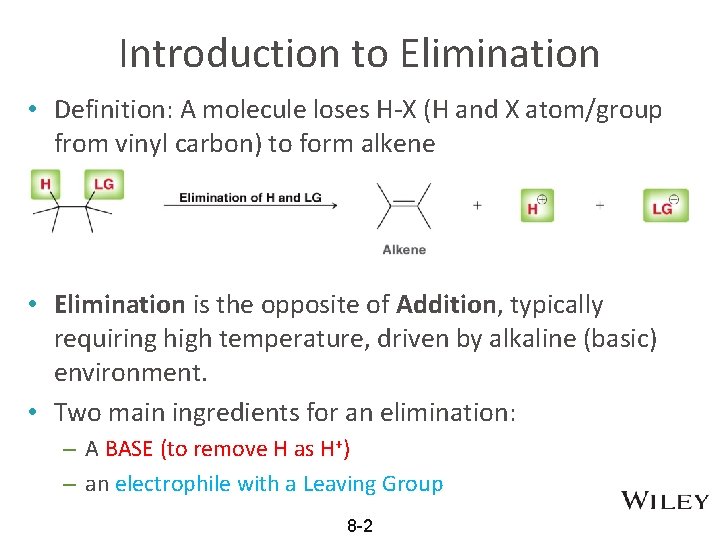
Introduction to Elimination • Definition: A molecule loses H-X (H and X atom/group from vinyl carbon) to form alkene • Elimination is the opposite of Addition, typically requiring high temperature, driven by alkaline (basic) environment. • Two main ingredients for an elimination: – A BASE (to remove H as H+) – an electrophile with a Leaving Group 8 -2

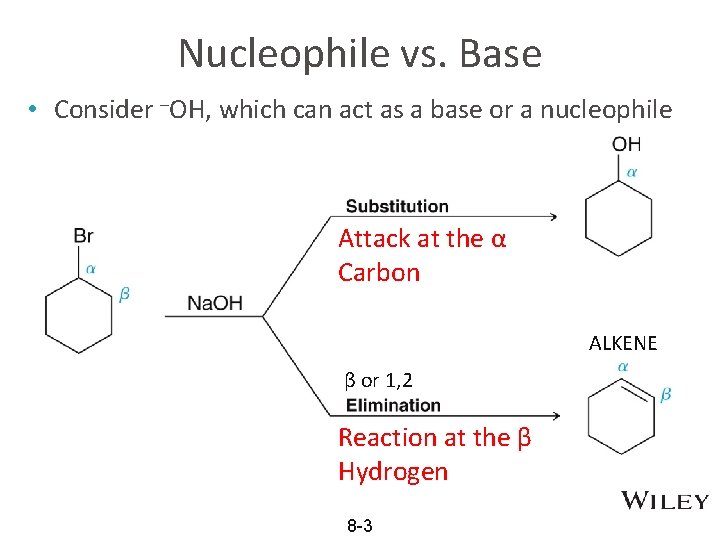
Nucleophile vs. Base • Consider –OH, which can act as a base or a nucleophile Attack at the α Carbon ALKENE β or 1, 2 Reaction at the β Hydrogen 8 -3

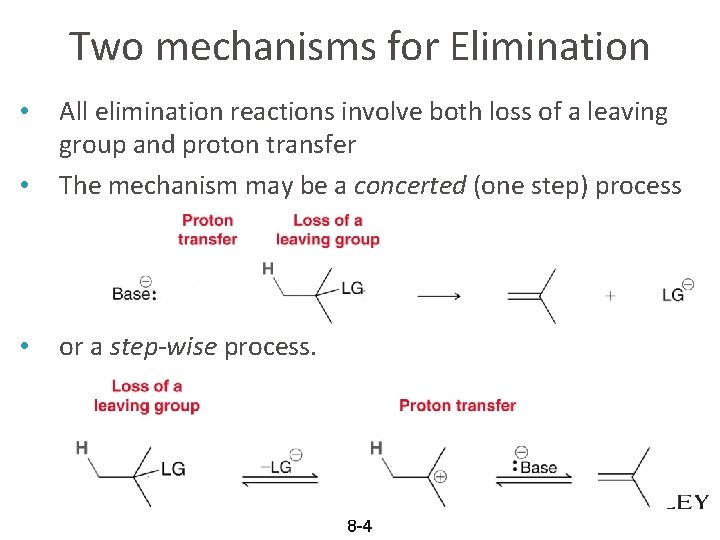
Two mechanisms for Elimination • All elimination reactions involve both loss of a leaving group and proton transfer The mechanism may be a concerted (one step) process • or a step-wise process. • 8 -4

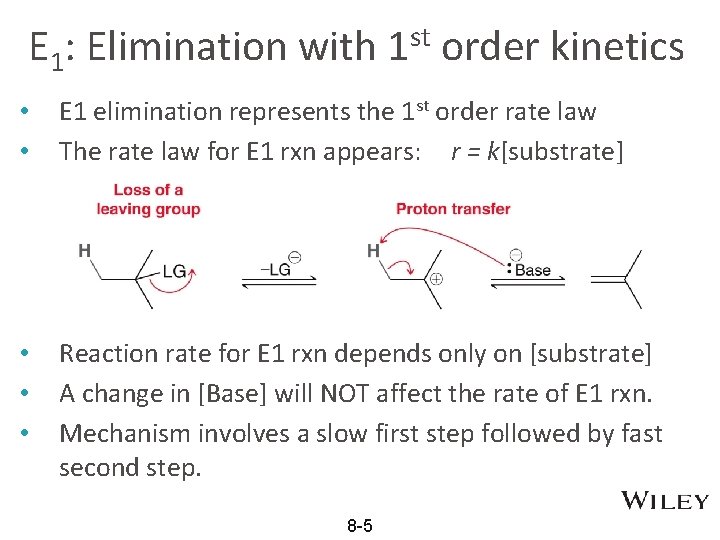
E 1: Elimination with 1 st order kinetics • • E 1 elimination represents the 1 st order rate law The rate law for E 1 rxn appears: r = k[substrate] • • • Reaction rate for E 1 rxn depends only on [substrate] A change in [Base] will NOT affect the rate of E 1 rxn. Mechanism involves a slow first step followed by fast second step. 8 -5

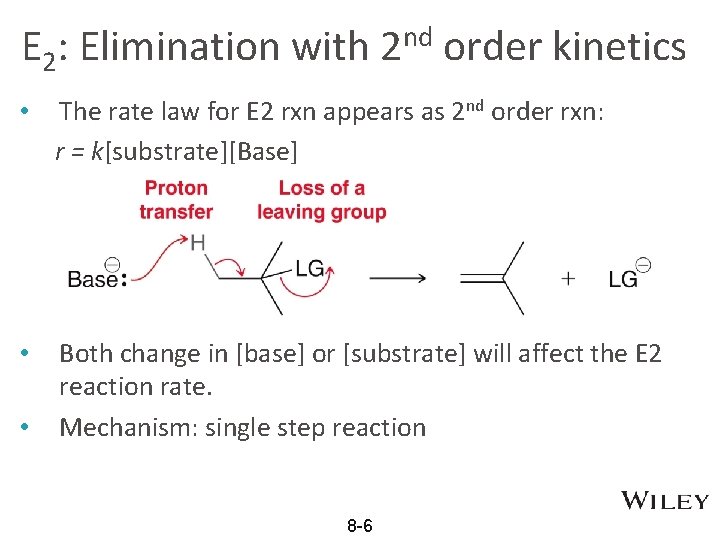
E 2: Elimination with 2 nd order kinetics • The rate law for E 2 rxn appears as 2 nd order rxn: r = k[substrate][Base] • Both change in [base] or [substrate] will affect the E 2 reaction rate. Mechanism: single step reaction • 8 -6

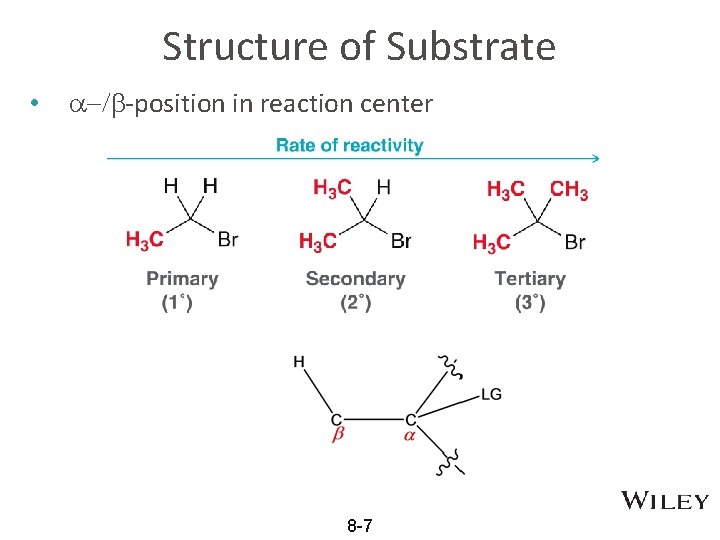
Structure of Substrate • a-/b-position in reaction center 8 -7

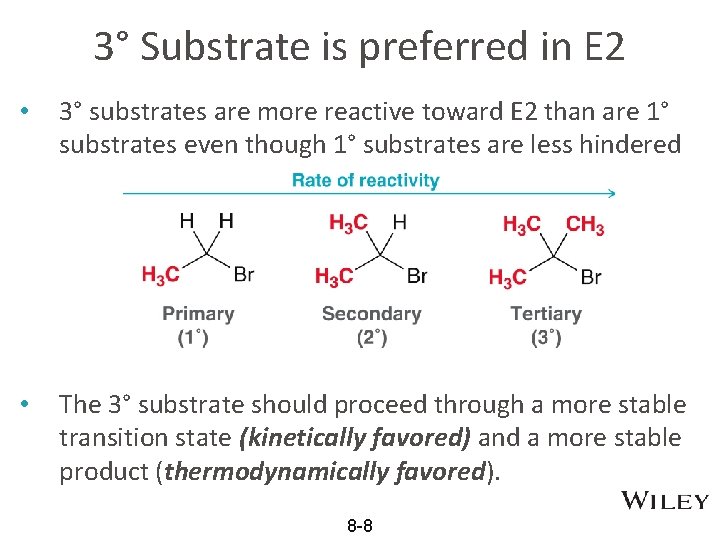
3° Substrate is preferred in E 2 • 3° substrates are more reactive toward E 2 than are 1° substrates even though 1° substrates are less hindered • The 3° substrate should proceed through a more stable transition state (kinetically favored) and a more stable product (thermodynamically favored). 8 -8

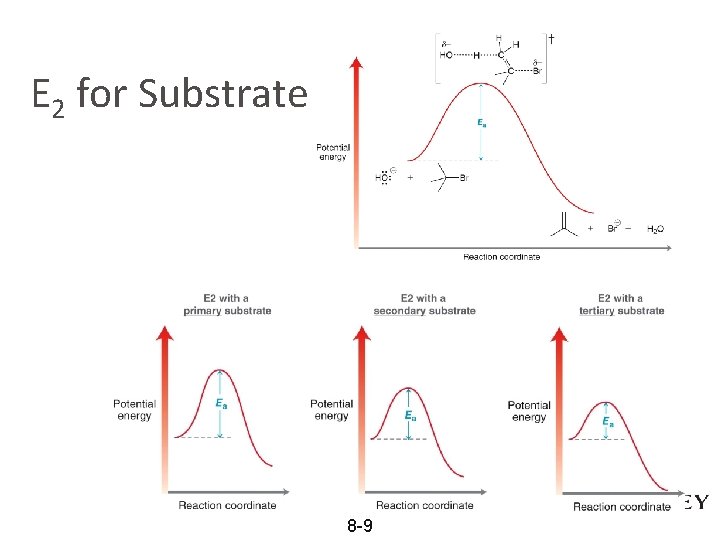
E 2 for Substrate 8 -9

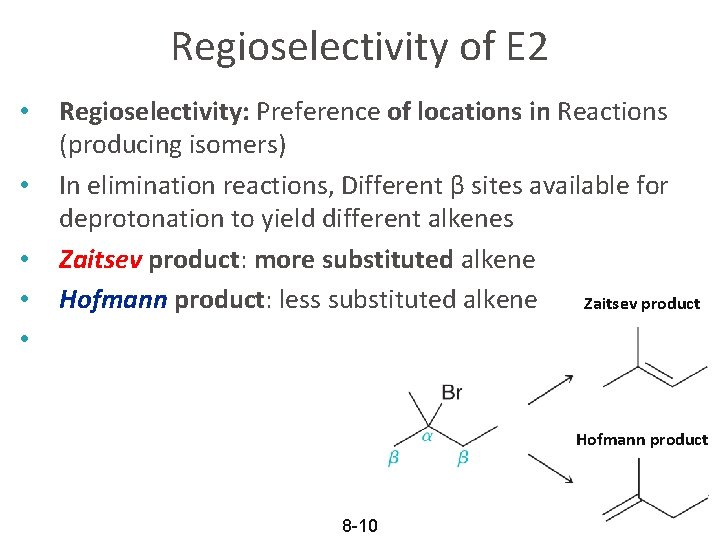
Regioselectivity of E 2 • • • Regioselectivity: Preference of locations in Reactions (producing isomers) In elimination reactions, Different β sites available for deprotonation to yield different alkenes Zaitsev product: more substituted alkene Hofmann product: less substituted alkene Zaitsev product Hofmann product 8 -10

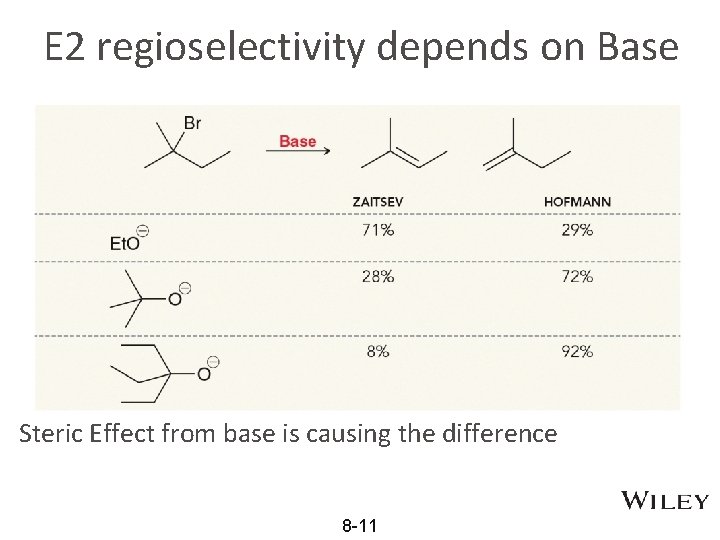
E 2 regioselectivity depends on Base Steric Effect from base is causing the difference 8 -11

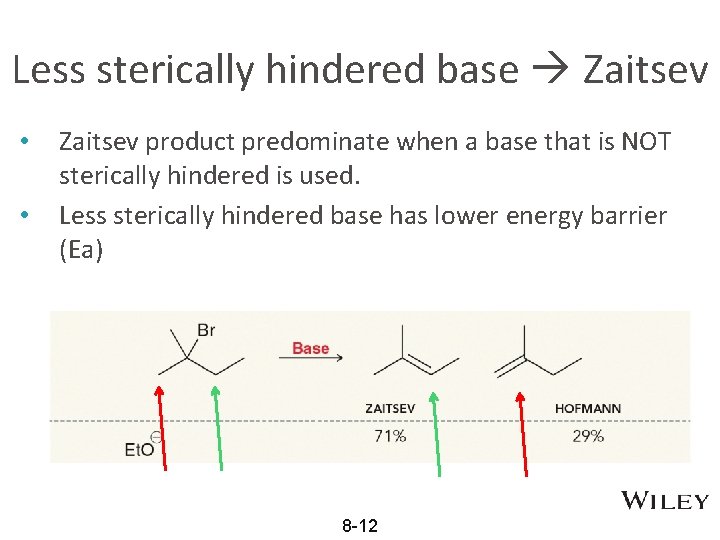
Less sterically hindered base Zaitsev • • Zaitsev product predominate when a base that is NOT sterically hindered is used. Less sterically hindered base has lower energy barrier (Ea) 8 -12

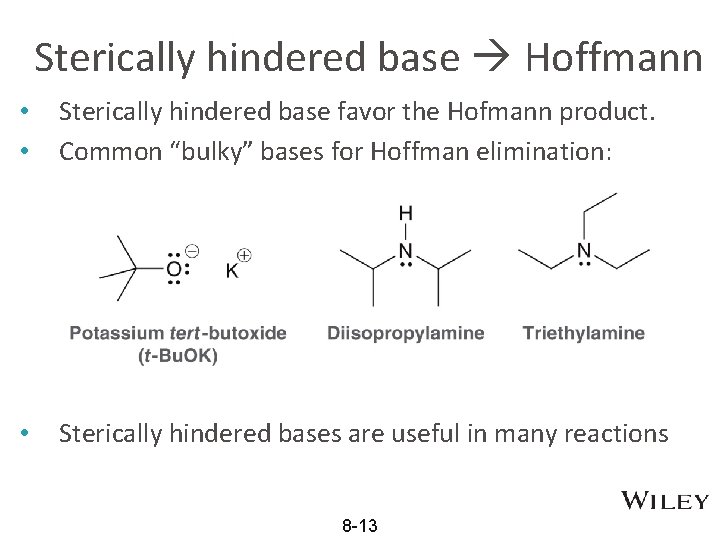
Sterically hindered base Hoffmann • • Sterically hindered base favor the Hofmann product. Common “bulky” bases for Hoffman elimination: • Sterically hindered bases are useful in many reactions 8 -13

Stereoselectivity of E 2 for Trans When two b-H atoms are available, dehydrohalogenation of 3 -bromopentane gives the following products Recall Trans isomer is more stable than cis isomer (thermodynamics) Trans has lower activation energy. 8 -14

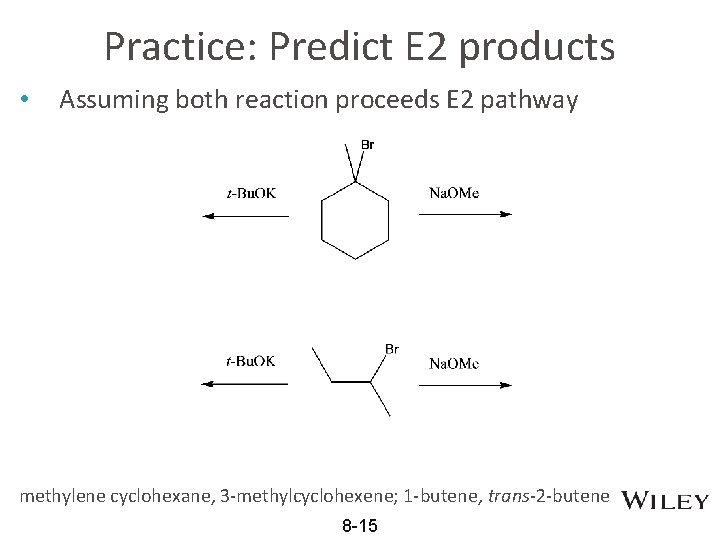
Practice: Predict E 2 products • Assuming both reaction proceeds E 2 pathway methylene cyclohexane, 3 -methylcyclohexene; 1 -butene, trans-2 -butene 8 -15

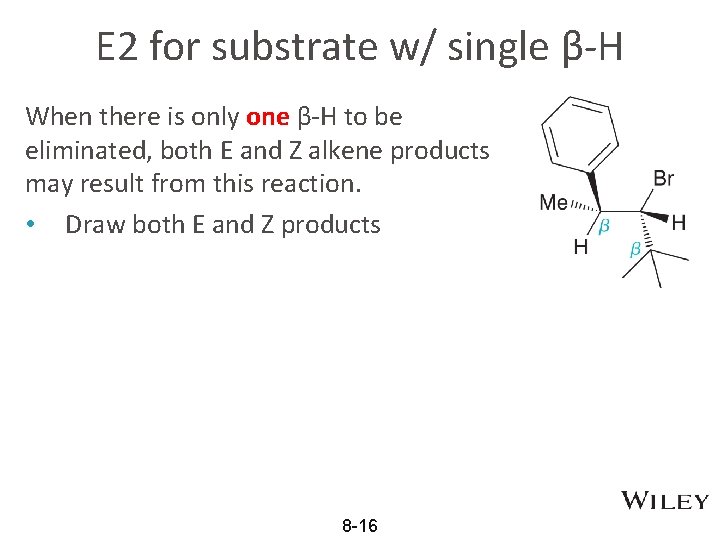
E 2 for substrate w/ single β-H When there is only one β-H to be eliminated, both E and Z alkene products may result from this reaction. • Draw both E and Z products 8 -16

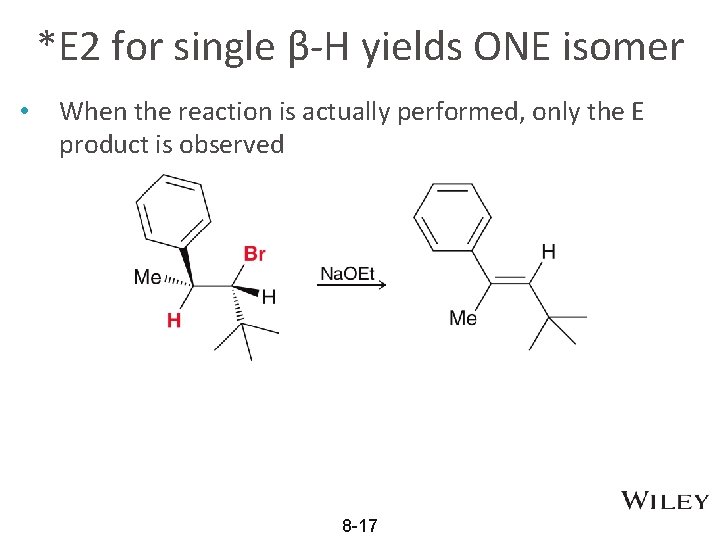
*E 2 for single β-H yields ONE isomer • When the reaction is actually performed, only the E product is observed 8 -17

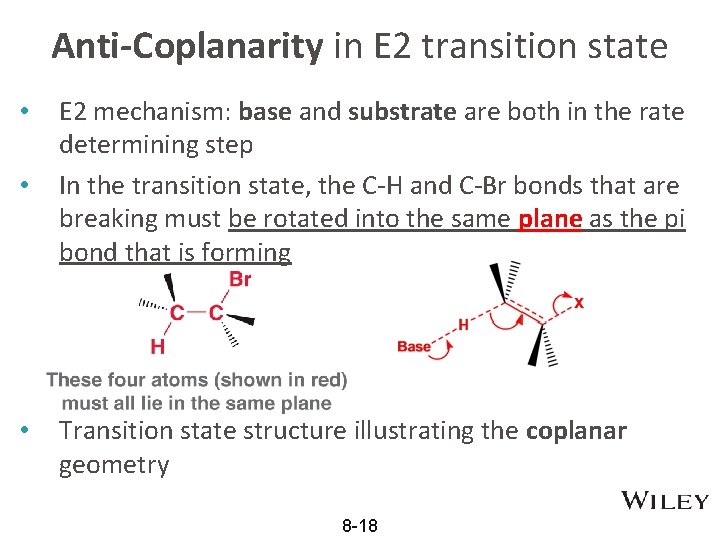
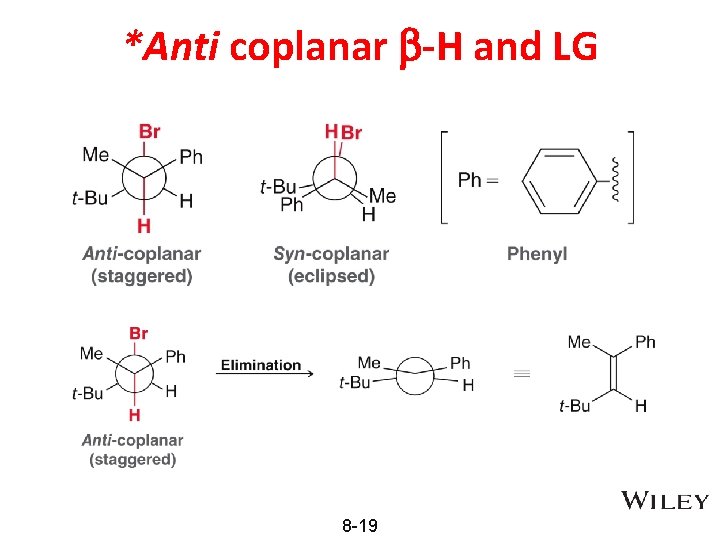
Anti-Coplanarity in E 2 transition state • • • E 2 mechanism: base and substrate are both in the rate determining step In the transition state, the C-H and C-Br bonds that are breaking must be rotated into the same plane as the pi bond that is forming Transition state structure illustrating the coplanar geometry 8 -18

*Anti coplanar b-H and LG 8 -19

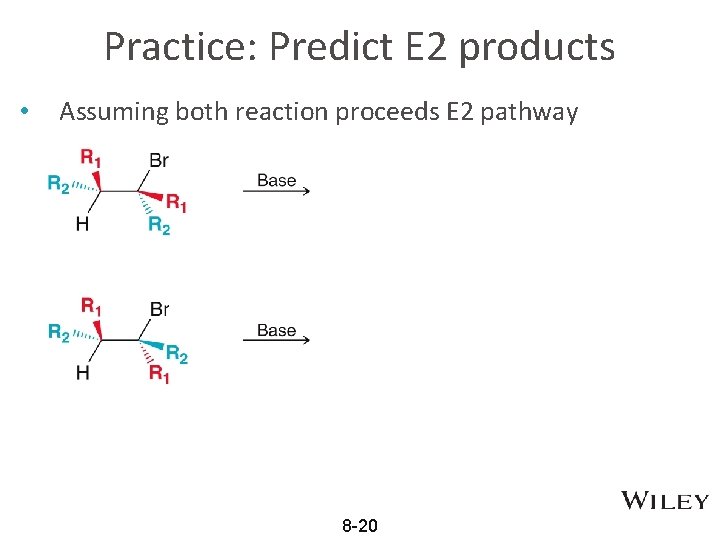
Practice: Predict E 2 products • Assuming both reaction proceeds E 2 pathway 8 -20

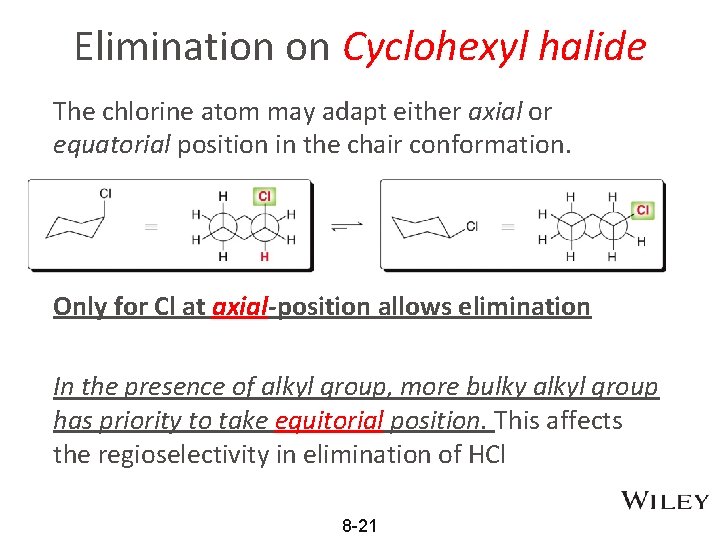
Elimination on Cyclohexyl halide The chlorine atom may adapt either axial or equatorial position in the chair conformation. Only for Cl at axial-position allows elimination In the presence of alkyl group, more bulky alkyl group has priority to take equitorial position. This affects the regioselectivity in elimination of HCl 8 -21

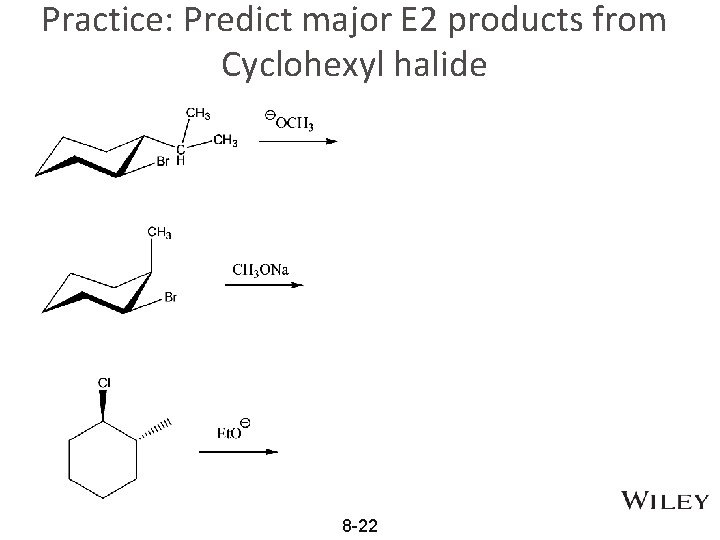
Practice: Predict major E 2 products from Cyclohexyl halide 8 -22

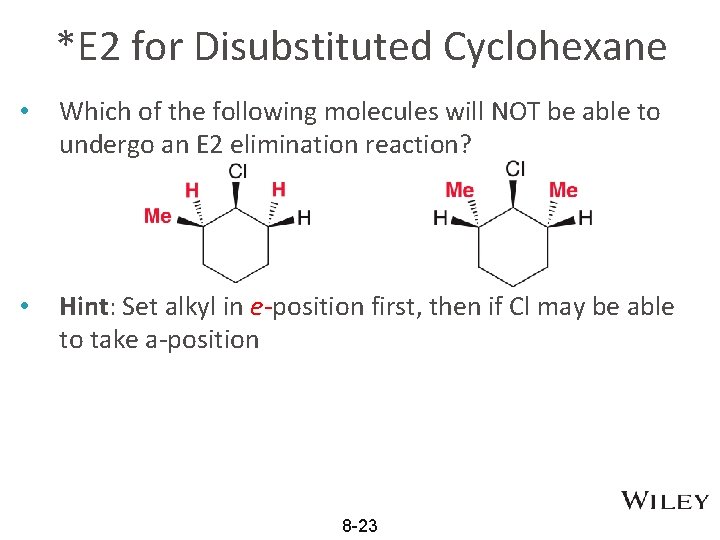
*E 2 for Disubstituted Cyclohexane • Which of the following molecules will NOT be able to undergo an E 2 elimination reaction? • Hint: Set alkyl in e-position first, then if Cl may be able to take a-position 8 -23

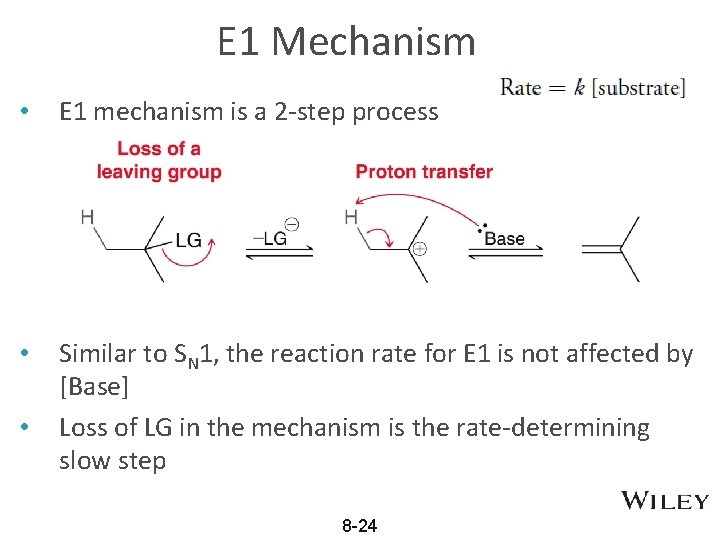
E 1 Mechanism • E 1 mechanism is a 2 -step process • Similar to SN 1, the reaction rate for E 1 is not affected by [Base] Loss of LG in the mechanism is the rate-determining slow step • 8 -24

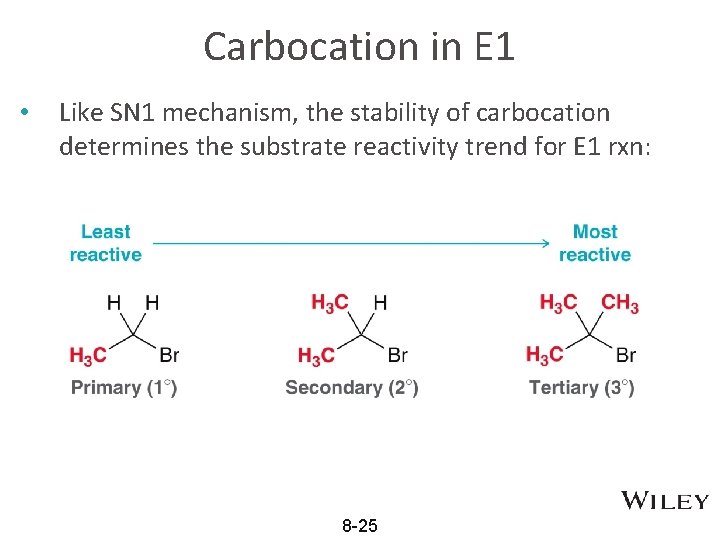
Carbocation in E 1 • Like SN 1 mechanism, the stability of carbocation determines the substrate reactivity trend for E 1 rxn: 8 -25

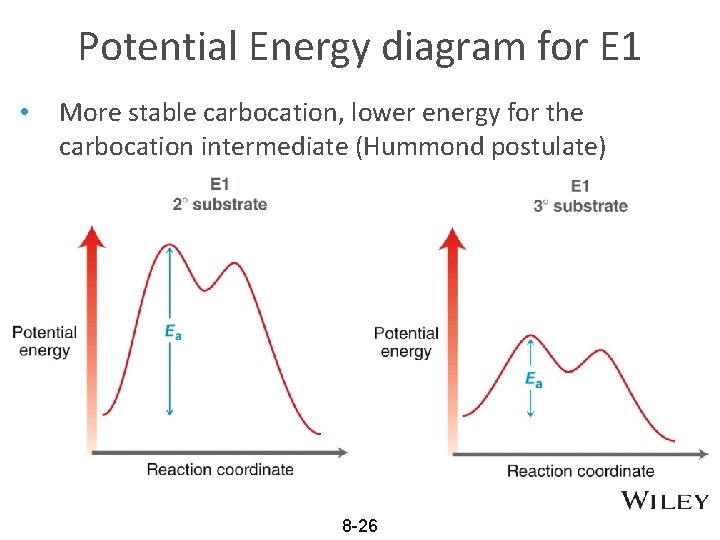
Potential Energy diagram for E 1 • More stable carbocation, lower energy for the carbocation intermediate (Hummond postulate) 8 -26

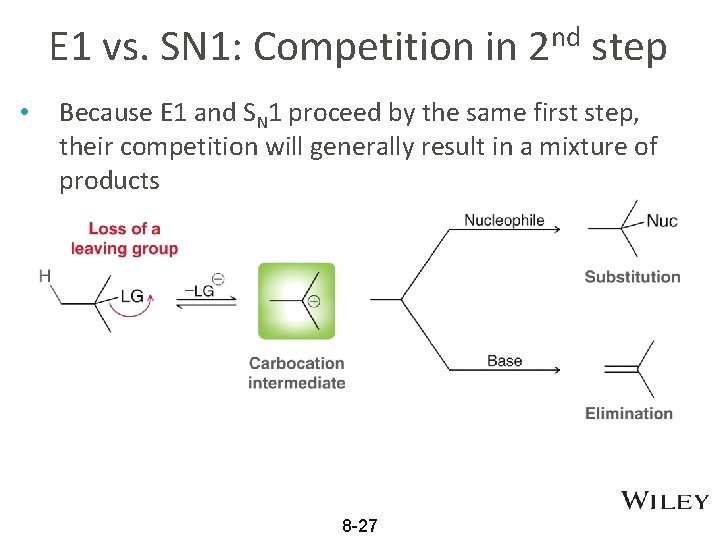
E 1 vs. SN 1: Competition in 2 nd step • Because E 1 and SN 1 proceed by the same first step, their competition will generally result in a mixture of products 8 -27

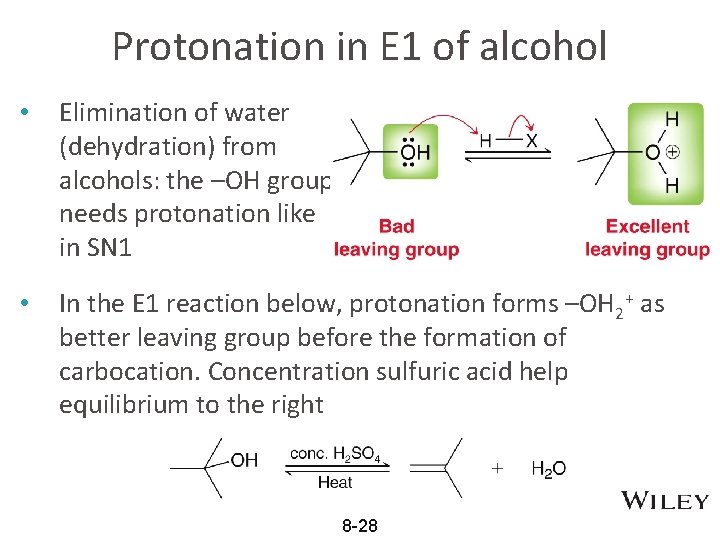
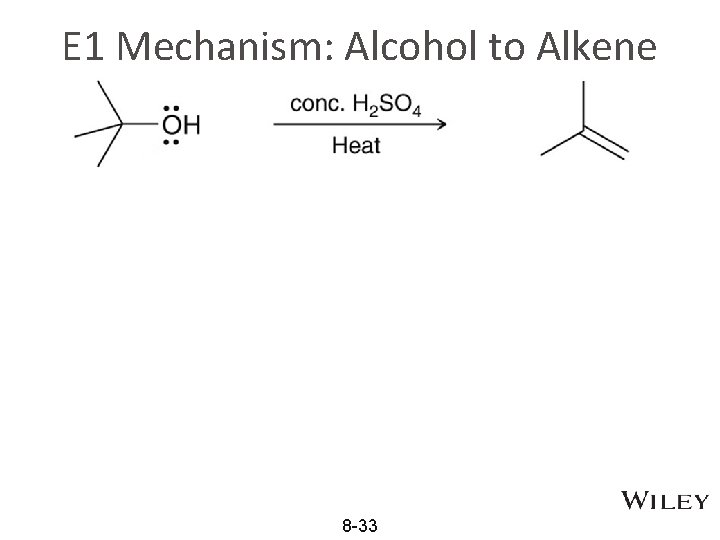
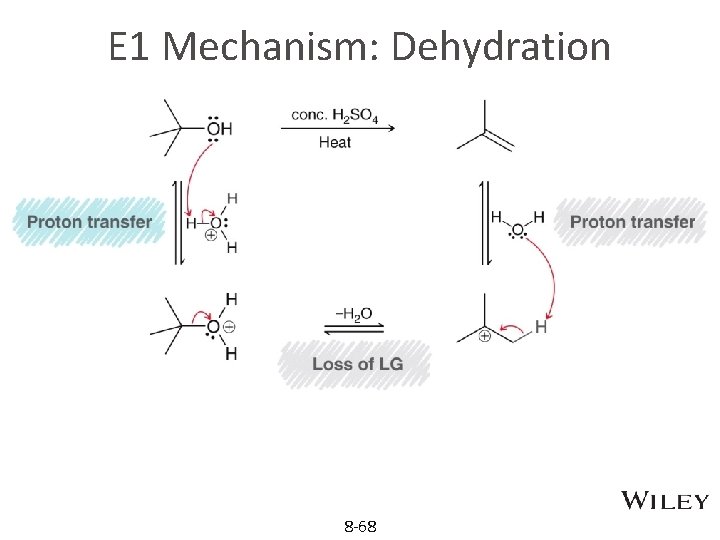
Protonation in E 1 of alcohol • Elimination of water (dehydration) from alcohols: the –OH group needs protonation like in SN 1 • In the E 1 reaction below, protonation forms –OH 2+ as better leaving group before the formation of carbocation. Concentration sulfuric acid help equilibrium to the right 8 -28

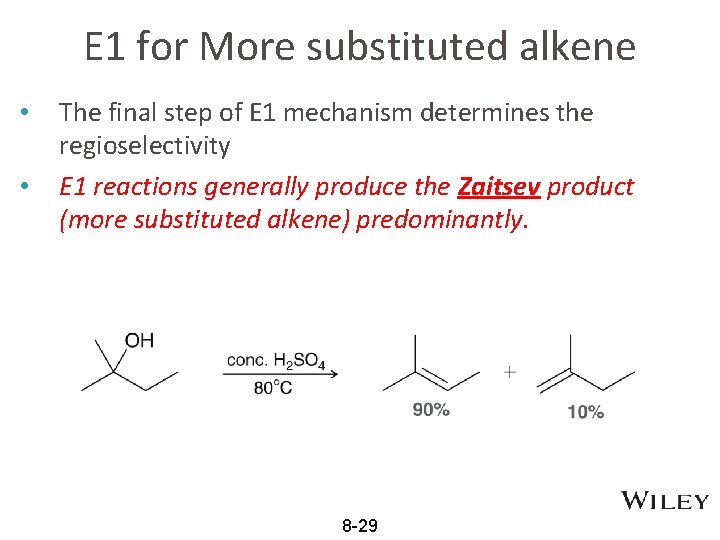
E 1 for More substituted alkene • • The final step of E 1 mechanism determines the regioselectivity E 1 reactions generally produce the Zaitsev product (more substituted alkene) predominantly. 8 -29

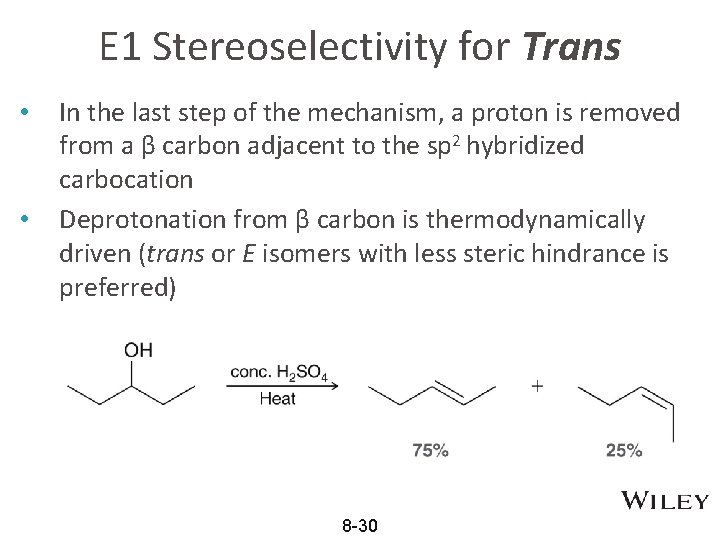
E 1 Stereoselectivity for Trans • • In the last step of the mechanism, a proton is removed from a β carbon adjacent to the sp 2 hybridized carbocation Deprotonation from β carbon is thermodynamically driven (trans or E isomers with less steric hindrance is preferred) 8 -30

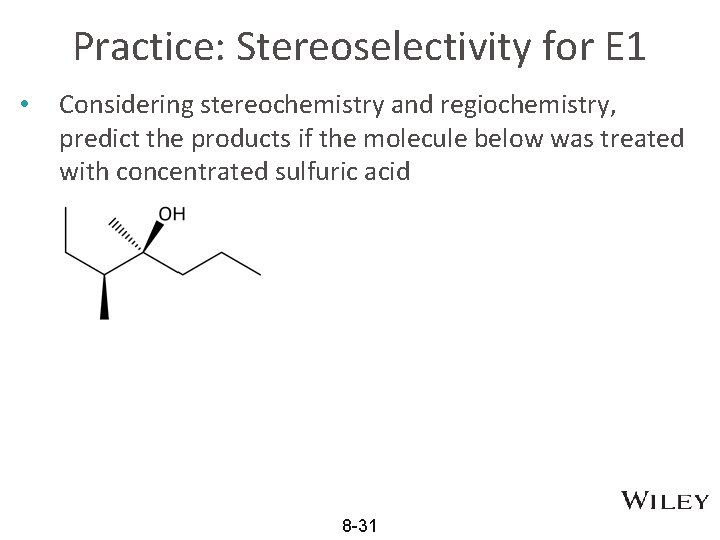
Practice: Stereoselectivity for E 1 • Considering stereochemistry and regiochemistry, predict the products if the molecule below was treated with concentrated sulfuric acid 8 -31

Complete E 1 Mechanisms • Recall the similarities between SN 1 and E 1 • After the carbocation is formed and possibly rearranged, E 1 proton transfer neutralizes the charge 8 -32

E 1 Mechanism: Alcohol to Alkene 8 -33

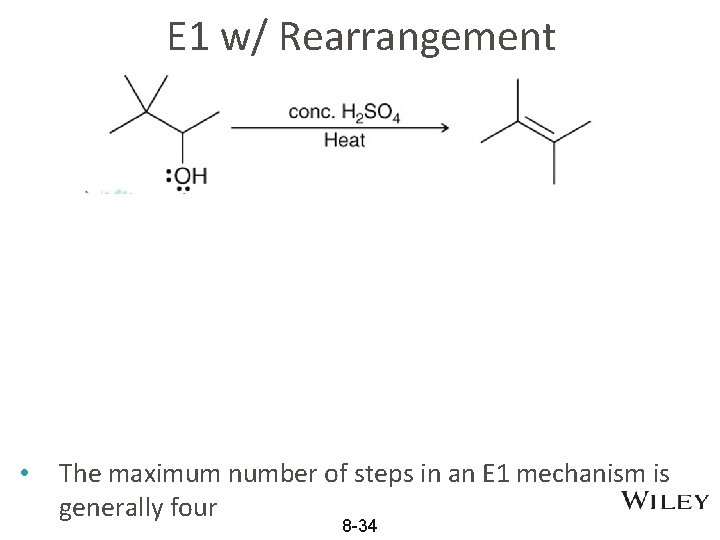
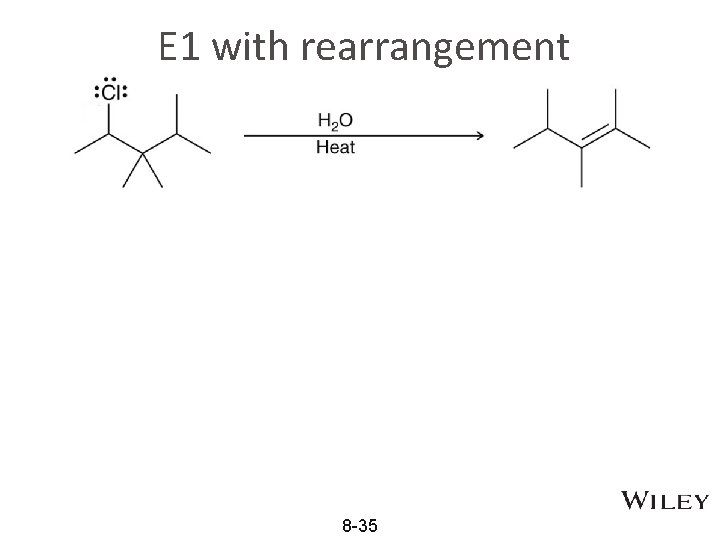
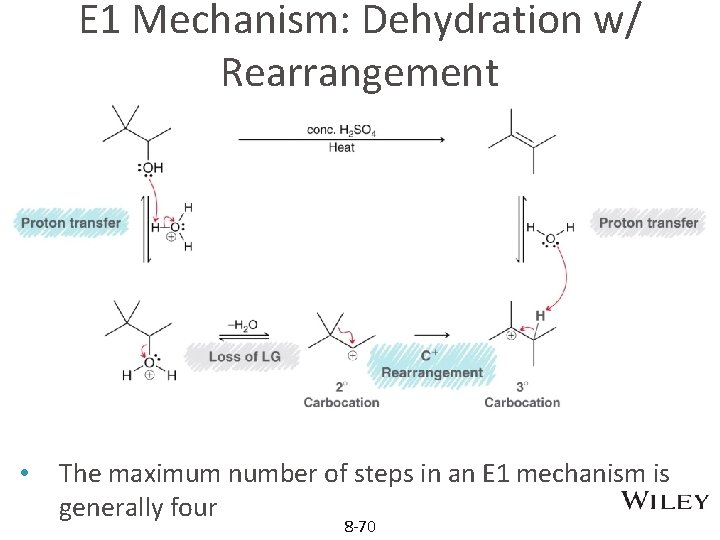
E 1 w/ Rearrangement • The maximum number of steps in an E 1 mechanism is generally four 8 -34

E 1 with rearrangement 8 -35

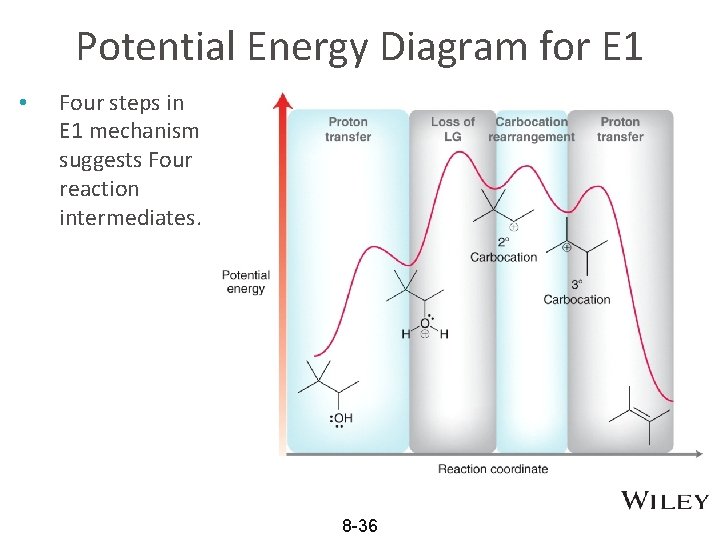
Potential Energy Diagram for E 1 • Four steps in E 1 mechanism suggests Four reaction intermediates. 8 -36

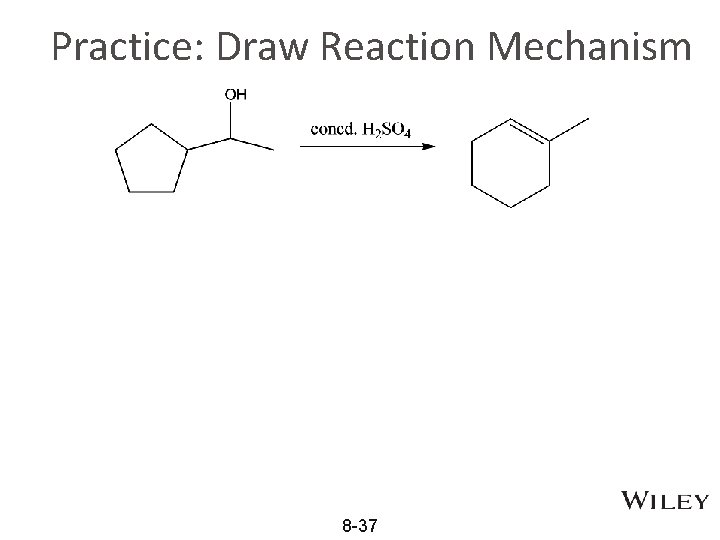
Practice: Draw Reaction Mechanism 8 -37

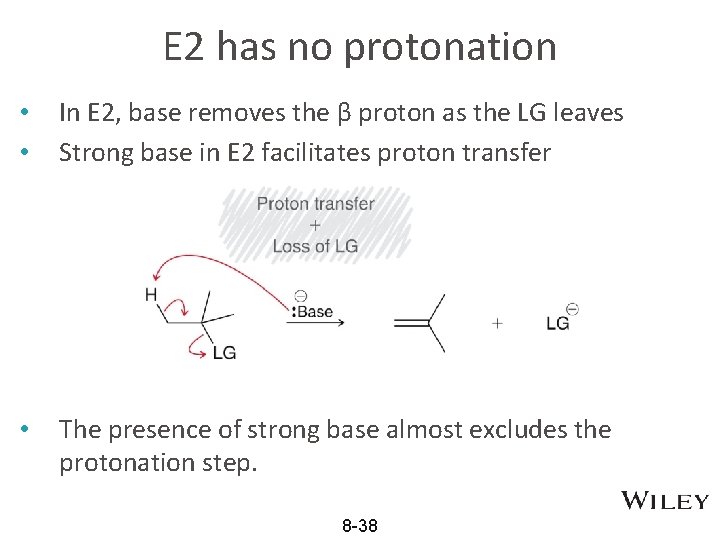
E 2 has no protonation • • In E 2, base removes the β proton as the LG leaves Strong base in E 2 facilitates proton transfer • The presence of strong base almost excludes the protonation step. 8 -38

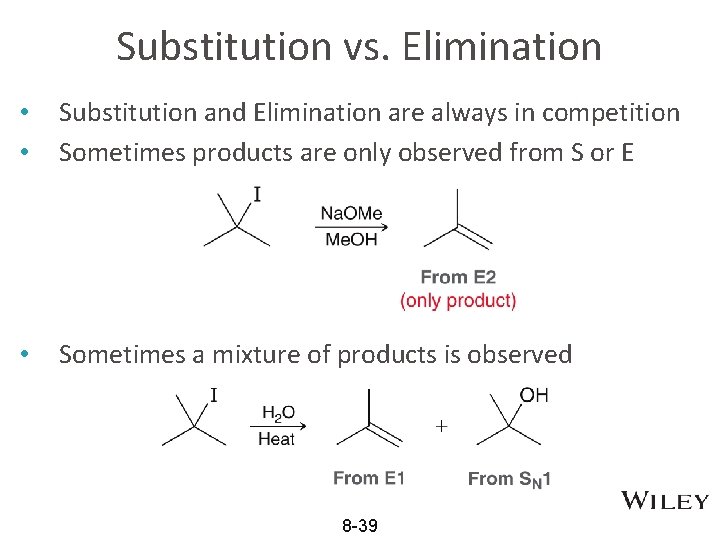
Substitution vs. Elimination • • Substitution and Elimination are always in competition Sometimes products are only observed from S or E • Sometimes a mixture of products is observed 8 -39

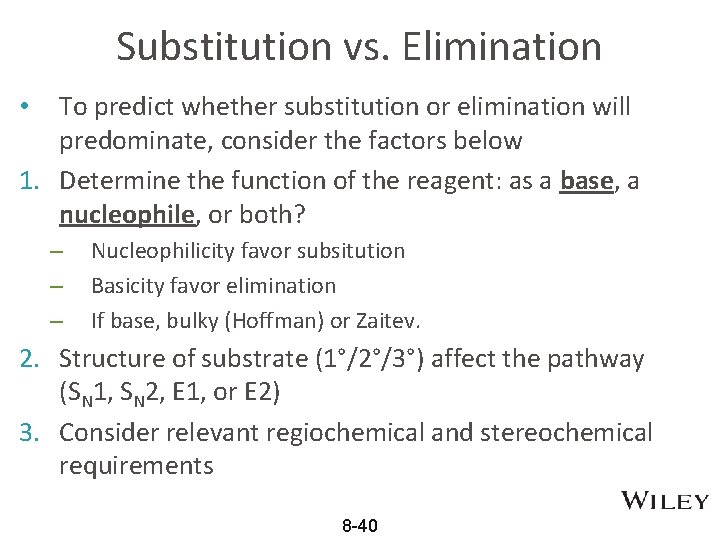
Substitution vs. Elimination To predict whether substitution or elimination will predominate, consider the factors below 1. Determine the function of the reagent: as a base, a nucleophile, or both? • – – – Nucleophilicity favor subsitution Basicity favor elimination If base, bulky (Hoffman) or Zaitev. 2. Structure of substrate (1°/2°/3°) affect the pathway (SN 1, SN 2, E 1, or E 2) 3. Consider relevant regiochemical and stereochemical requirements 8 -40

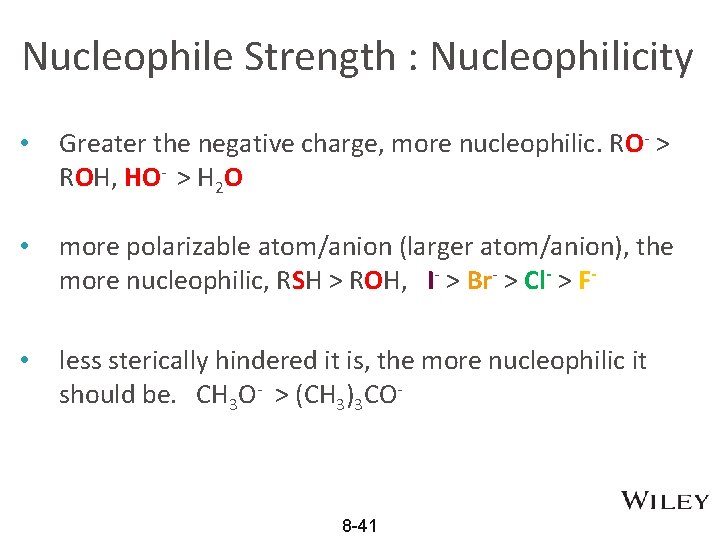
Nucleophile Strength : Nucleophilicity • Greater the negative charge, more nucleophilic. RO- > ROH, HO- > H 2 O • more polarizable atom/anion (larger atom/anion), the more nucleophilic, RSH > ROH, I- > Br- > Cl- > F- • less sterically hindered it is, the more nucleophilic it should be. CH 3 O- > (CH 3)3 CO- 8 -41

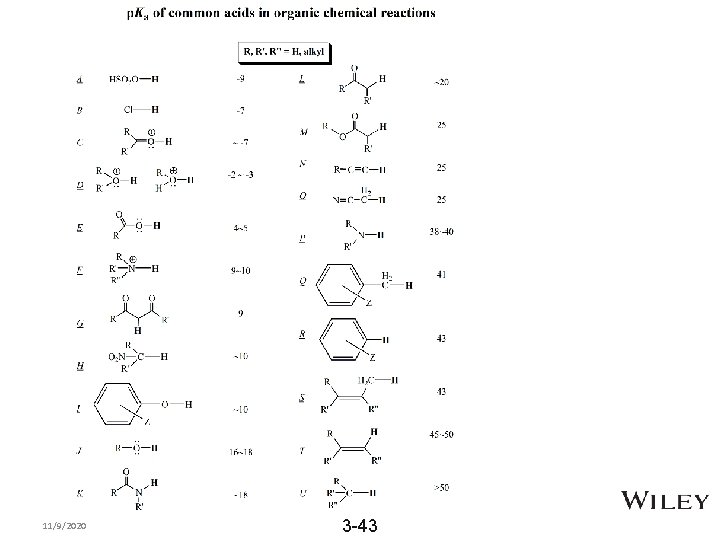
Strength of Base: Basicity • • Recall strong acid (low p. Ka ) yields weak conjugate base Strong base is the conjugate base from weak acid • To predict the basicity of base, add H+ to make conjugate acid then use ARIO (atom, resonance, induction, orbital) to predict acidicity. Compare the following: • – CH 3 OH or CH 3 NH 2 – Acetate or RO- 8 -42

11/9/2020 3 -43

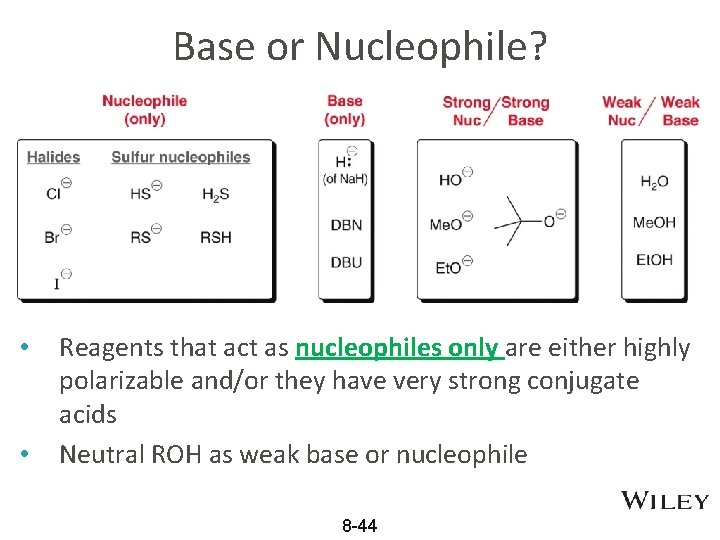
Base or Nucleophile? • • Reagents that act as nucleophiles only are either highly polarizable and/or they have very strong conjugate acids Neutral ROH as weak base or nucleophile 8 -44

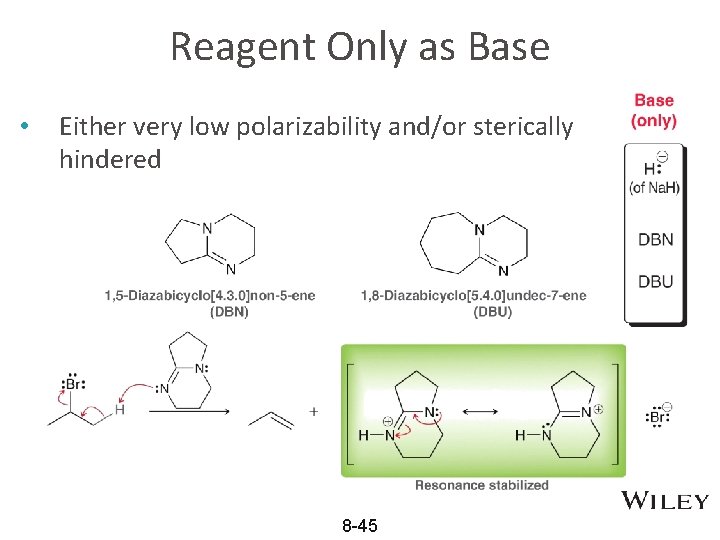
Reagent Only as Base • Either very low polarizability and/or sterically hindered 8 -45

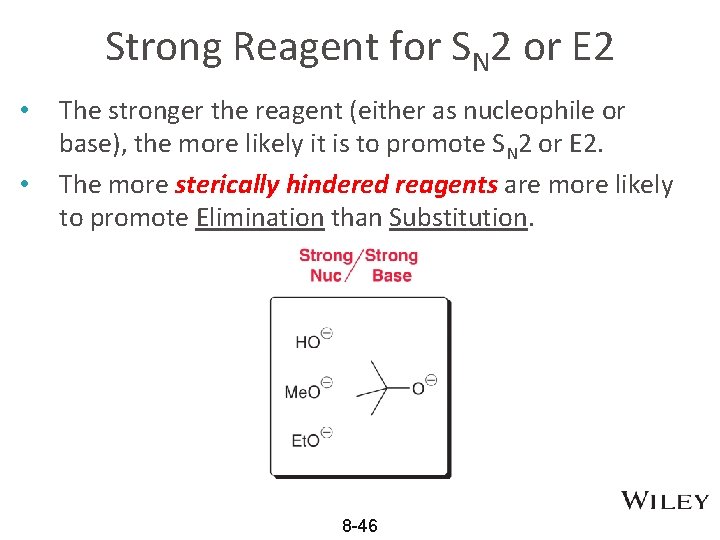
Strong Reagent for SN 2 or E 2 • • The stronger the reagent (either as nucleophile or base), the more likely it is to promote SN 2 or E 2. The more sterically hindered reagents are more likely to promote Elimination than Substitution. 8 -46

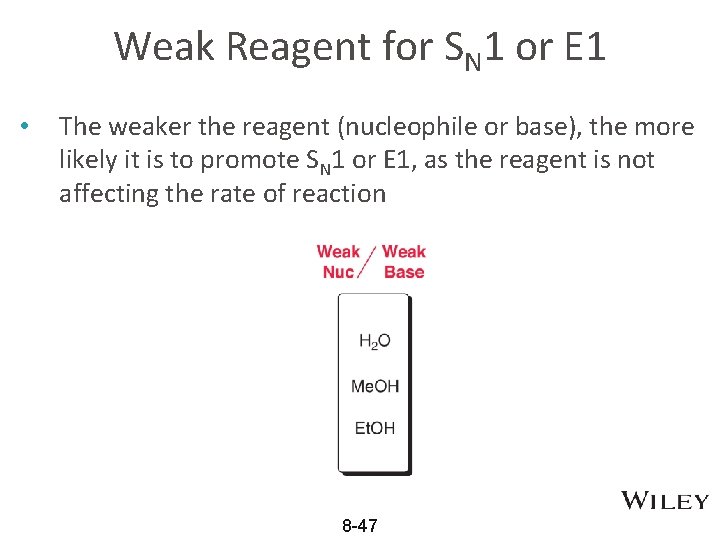
Weak Reagent for SN 1 or E 1 • The weaker the reagent (nucleophile or base), the more likely it is to promote SN 1 or E 1, as the reagent is not affecting the rate of reaction 8 -47

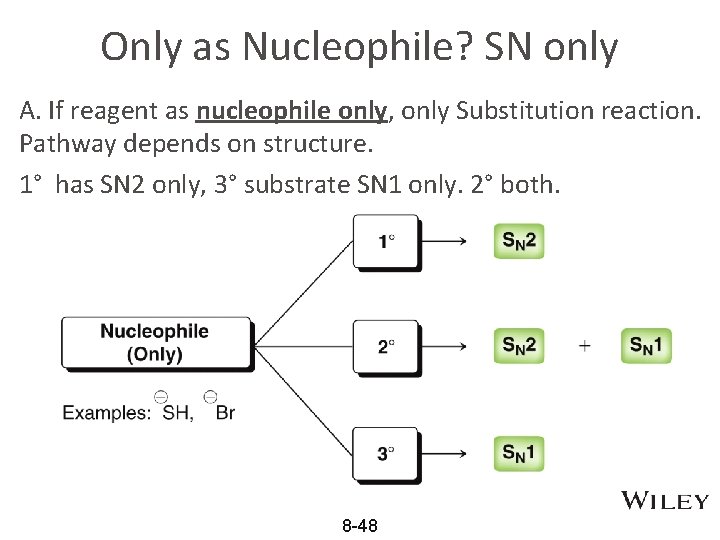
Only as Nucleophile? SN only A. If reagent as nucleophile only, only Substitution reaction. Pathway depends on structure. 1° has SN 2 only, 3° substrate SN 1 only. 2° both. 8 -48

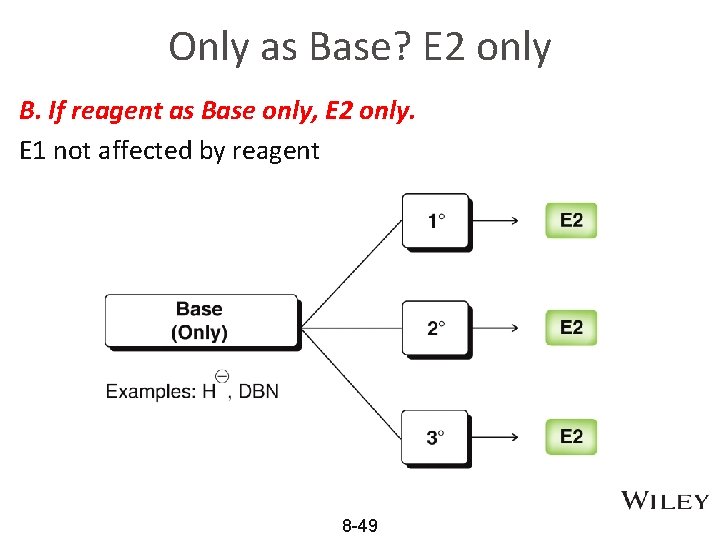
Only as Base? E 2 only B. If reagent as Base only, E 2 only. E 1 not affected by reagent 8 -49

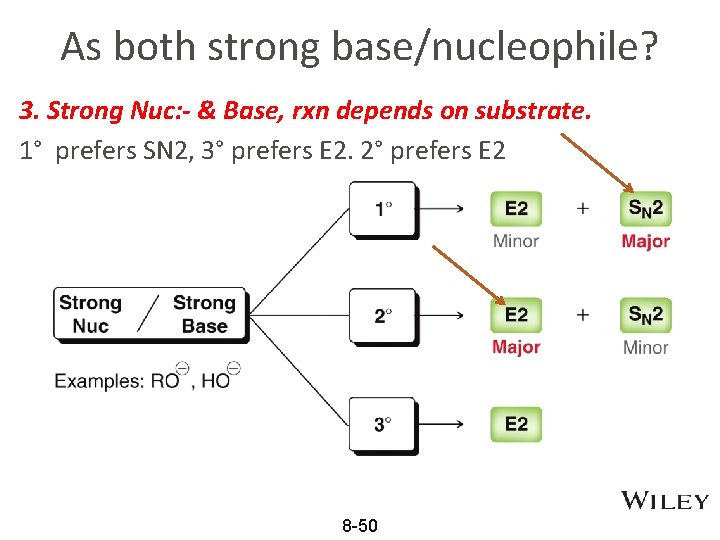
As both strong base/nucleophile? 3. Strong Nuc: - & Base, rxn depends on substrate. 1° prefers SN 2, 3° prefers E 2. 2° prefers E 2 8 -50

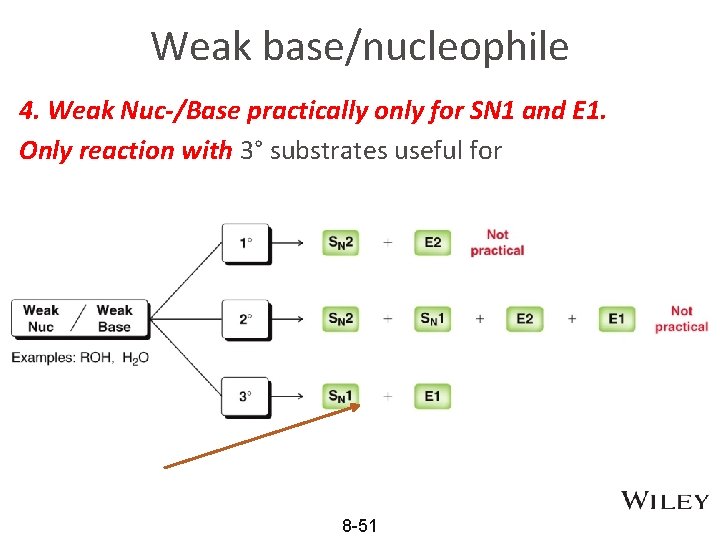
Weak base/nucleophile 4. Weak Nuc-/Base practically only for SN 1 and E 1. Only reaction with 3° substrates useful for • Practice with Skill. Builder 8. 11 8 -51

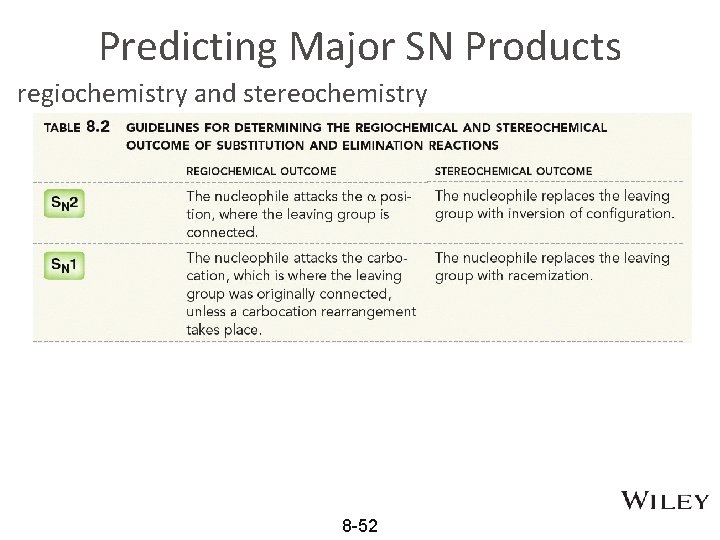
Predicting Major SN Products regiochemistry and stereochemistry 8 -52

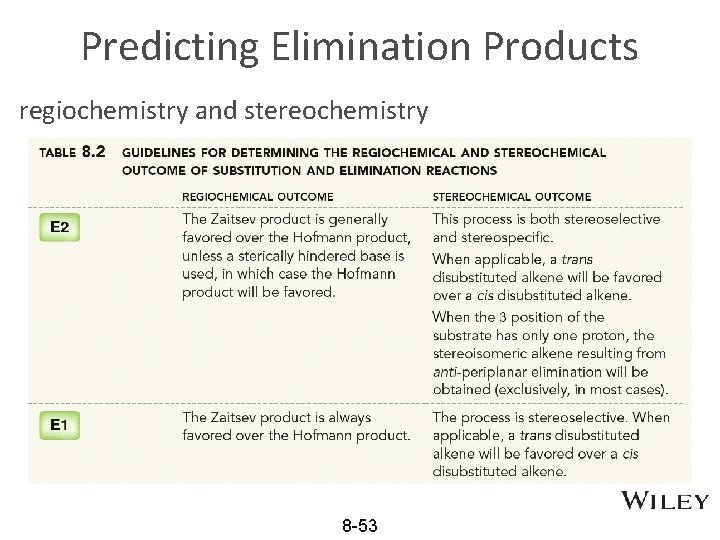
Predicting Elimination Products regiochemistry and stereochemistry 8 -53

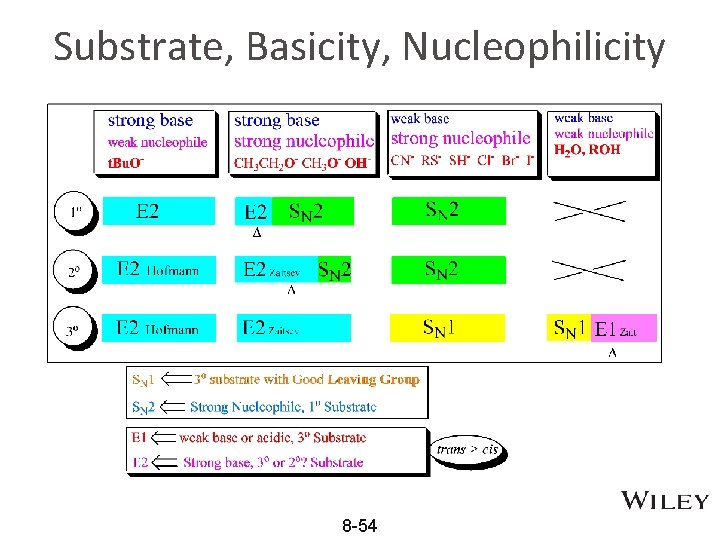
Substrate, Basicity, Nucleophilicity 8 -54

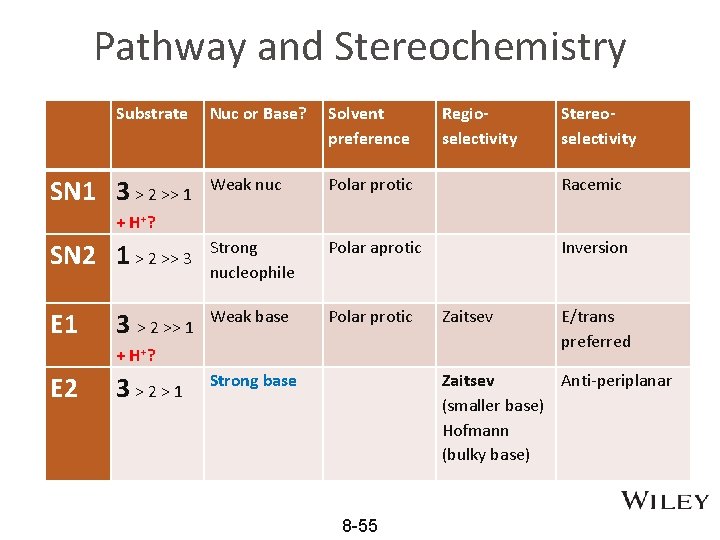
Pathway and Stereochemistry Nuc or Base? Solvent preference Regioselectivity Stereoselectivity Weak nuc Polar protic Racemic SN 2 1 > 2 >> 3 Strong nucleophile Polar aprotic Inversion E 1 Weak base Polar protic Zaitsev E/trans preferred Strong base Zaitsev Anti-periplanar (smaller base) Hofmann (bulky base) Substrate SN 1 3 > 2 >> 1 + H+? E 2 3 > 2 > 1 8 -55

Additional Practice Problems • For the substrate, give both the kinetically favored E 2 product and thermodynamically favored E 2 product. Explain what conditions can be used to favor each. 8 -56

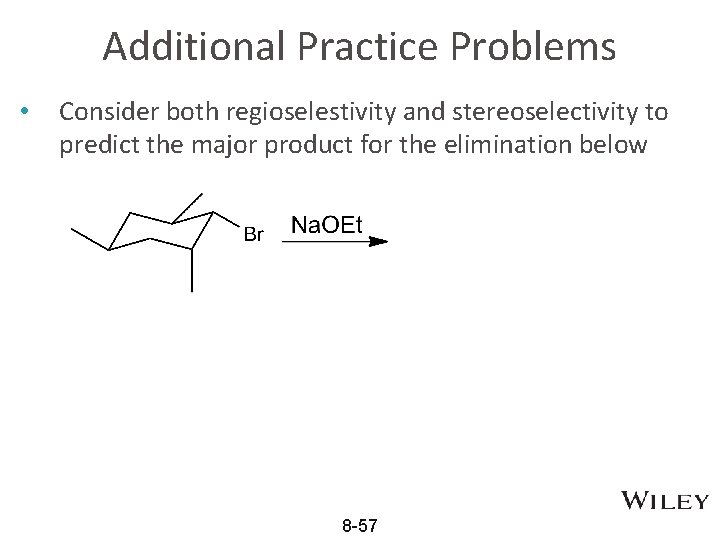
Additional Practice Problems • Consider both regioselestivity and stereoselectivity to predict the major product for the elimination below 8 -57

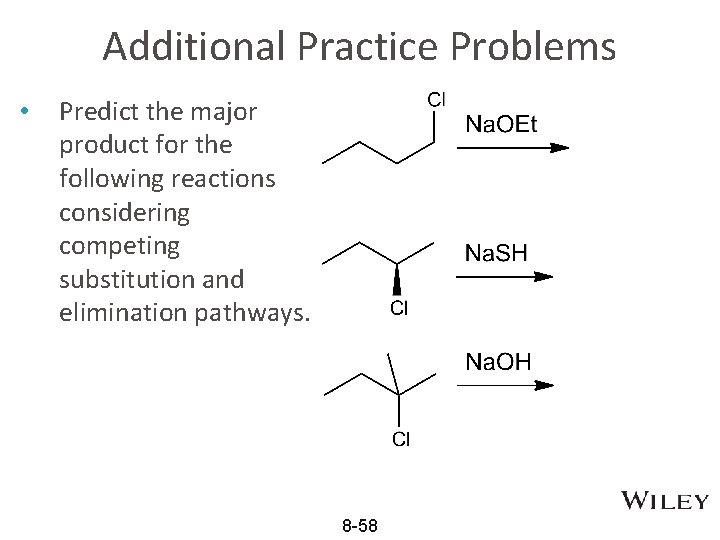
Additional Practice Problems • Predict the major product for the following reactions considering competing substitution and elimination pathways. 8 -58

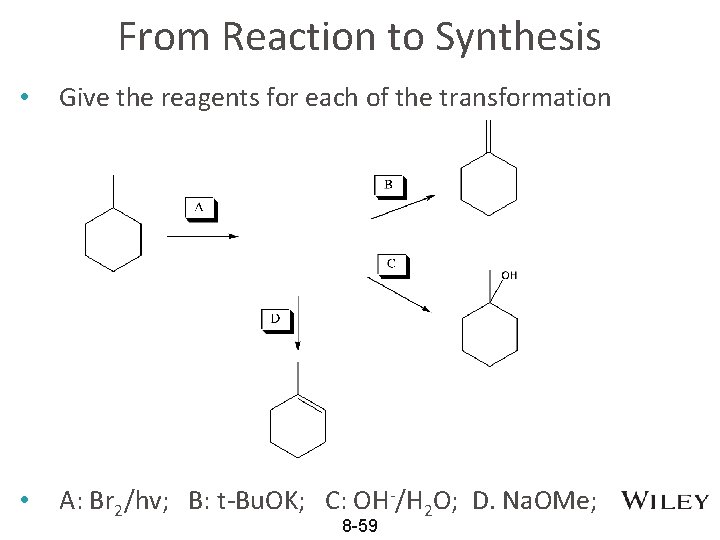
From Reaction to Synthesis • Give the reagents for each of the transformation • A: Br 2/hv; B: t-Bu. OK; C: OH-/H 2 O; D. Na. OMe; 8 -59

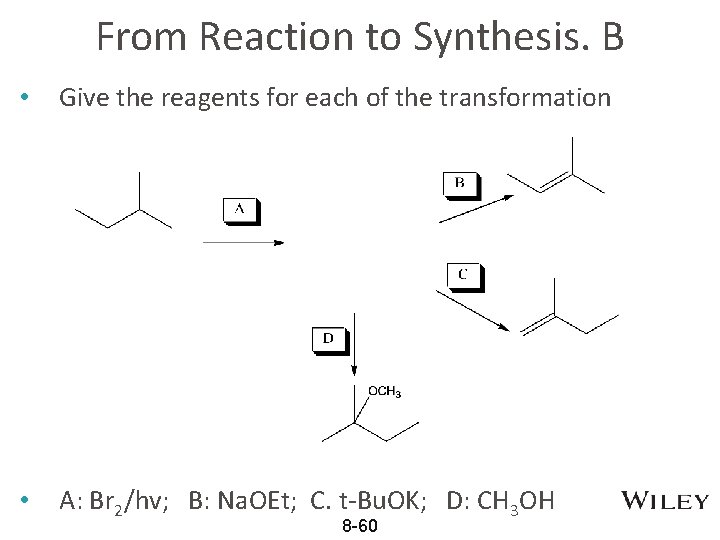
From Reaction to Synthesis. B • Give the reagents for each of the transformation • A: Br 2/hv; B: Na. OEt; C. t-Bu. OK; D: CH 3 OH 8 -60

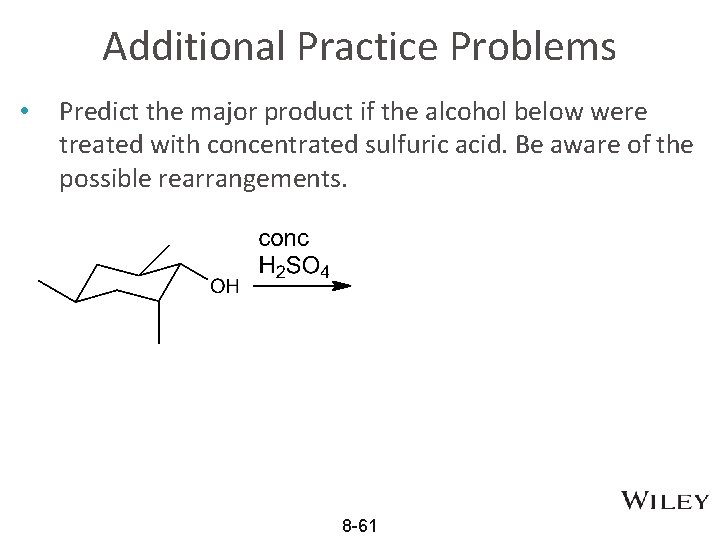
Additional Practice Problems • Predict the major product if the alcohol below were treated with concentrated sulfuric acid. Be aware of the possible rearrangements. 8 -61

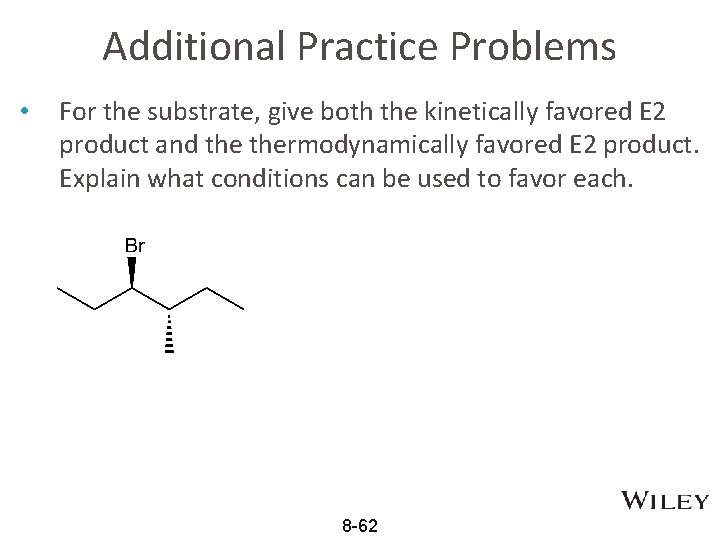
Additional Practice Problems • For the substrate, give both the kinetically favored E 2 product and thermodynamically favored E 2 product. Explain what conditions can be used to favor each. 8 -62

Additional Practice Problems • Since tertiary substrates react more readily than secondary or primary in both E 1 and E 2 mechanisms, what factor(s) usually controls which mechanism will dominate and why? 8 -63

**Anti-periplanar transition state for E 2 • • • Experiments suggest that a strict 180° angle is NOT necessary for E 2 mechanisms. Substituents on a and b carbon might have gauche interaction when achieving anti-coplanarity Similar angles (175– 179°) are sufficient (anti-periplanar instead of anti-coplanar) Thus even when E isomer is usually more stable, the requirement for an anti-periplanar transition state can often lead to the less stable Z isomer. Thus the rxn is NOT thermodynamically driven, rather kinetically driven. 8 -64

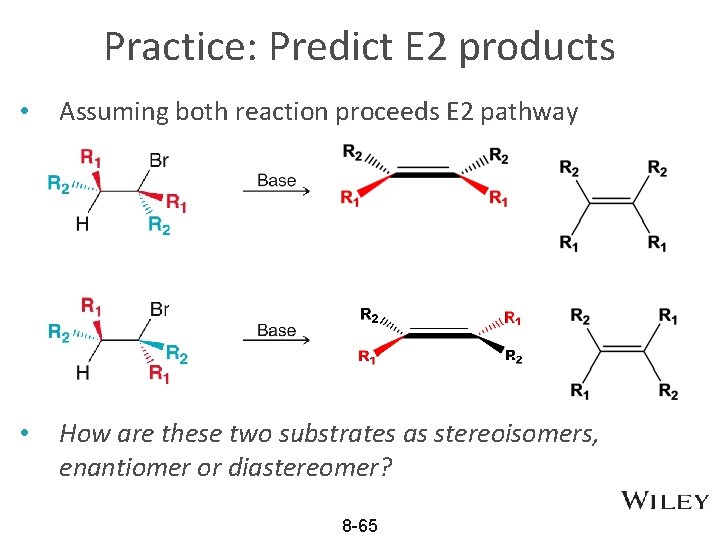
Practice: Predict E 2 products • Assuming both reaction proceeds E 2 pathway • How are these two substrates as stereoisomers, enantiomer or diastereomer? 8 -65

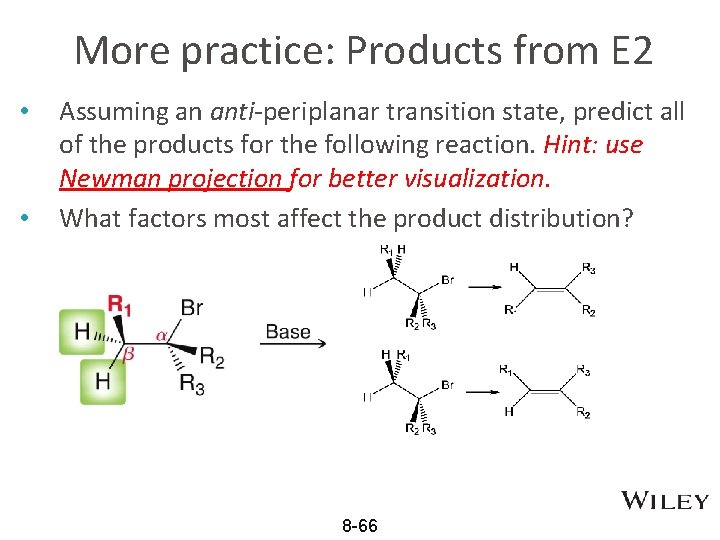
More practice: Products from E 2 • • Assuming an anti-periplanar transition state, predict all of the products for the following reaction. Hint: use Newman projection for better visualization. What factors most affect the product distribution? 8 -66

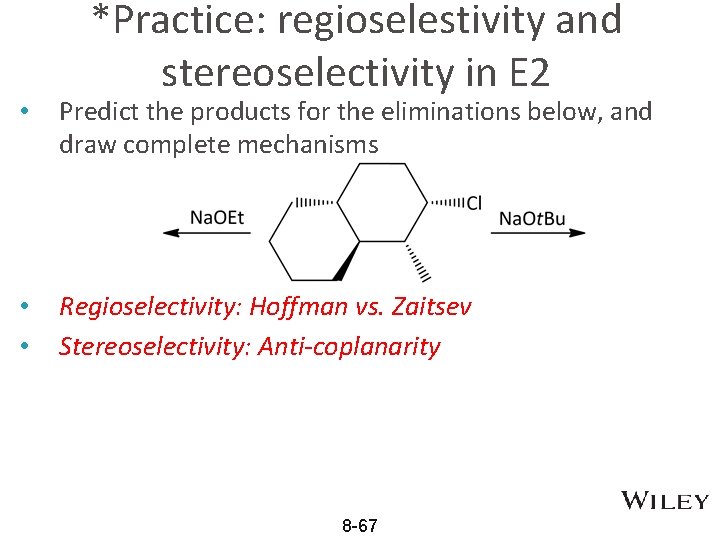
*Practice: regioselestivity and stereoselectivity in E 2 • Predict the products for the eliminations below, and draw complete mechanisms • • Regioselectivity: Hoffman vs. Zaitsev Stereoselectivity: Anti-coplanarity 8 -67

E 1 Mechanism: Dehydration 8 -68

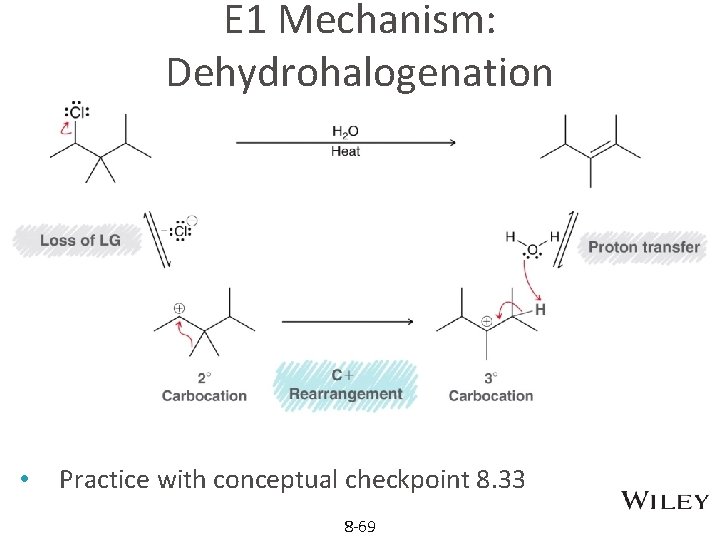
E 1 Mechanism: Dehydrohalogenation • Practice with conceptual checkpoint 8. 33 8 -69

E 1 Mechanism: Dehydration w/ Rearrangement • The maximum number of steps in an E 1 mechanism is generally four 8 -70