Elements Their Properties Chapter 17 Properties of Metals





















- Slides: 26

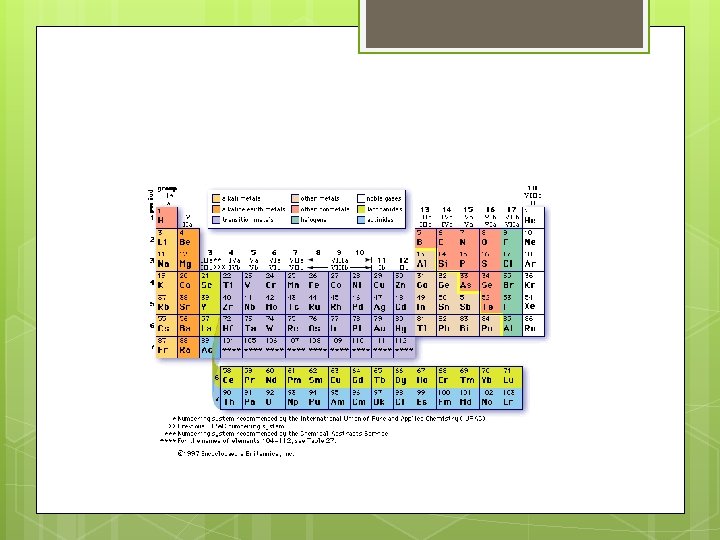
Elements & Their Properties Chapter 17

Properties of Metals Conduct heat & electricity Luster: reflects light well Malleable: can be hammered or rolled into sheets Ductile: can be drawn into wires

Properties of Metals Ionic bonding: combine with nonmetals by losing electrons Metallic bonding: positively charged metallic ions are surrounded by a cloud of electrons; ions are in sliding layers & electrons are weakly held; readily form ionic bonds with nonmetals


Group 1 except Hydrogen The Alkali Metals Softer & more reactive than other metals Highly reactive with oxygen & water Don’t occur naturally as elemental forms Combine readily with other elements due to a single electron in the outer energy level

Group 1 except Hydrogen The Alkali Metals Multiple uses Human health: sodium, potassium & lithium compounds Photocells: some depend on rubidium or cesium Francium: a radioactive element which breaks down, giving off particles and energy

Group 2 The Alkaline Earth Metals Not found naturally in elemental form; two electrons in the outer energy layer Applications: Strontium and magnesium found in fireworks Magnesium in vehicles, ladders and bats Calcium in statues and countertops Human body: Calcium in bones Barium in disease diagnoses Radium formerly used in cancer treatment

Groups 3 -12 Transition Elements Often occur in nature as uncombined elements Form colored compounds Chromium Iron found in rubies & emeralds triad: iron, cobalt, nickel Iron: most widely used metal & main ingredient in steel; abundant in earth’s crust Cobalt & nickel: used in some steels Nickel: used to coat other metals

Groups 3 -12 Transition Elements Copper, silver, gold Coinage metals (used to make coins) Copper: used in electric wiring because it is of electricity Silver: used in photographic film & paper; Gold: used in jewelry a superior conductor jewelry

Groups 3 -12 The Transition Elements Zinc, cadmium, mercury Group Zinc 12 on the periodic table & cadmium: used to coat other metals Mercury: only room temperature liquid metal; used in thermometers and batteries

The Inner Transition Metals Seen disconnected from the rest of the table Lanthanides: includes lanthanum, cerium, praseodymium, amarium, europium, gadolinium, and terbium Actinides: all are radioactive and unstable; uranium is the best known

Properties Usually of Nonmetals gases or brittle solids at room temperature Not malleable or ductile Poor conductors Not lustrous Make up most of the human body

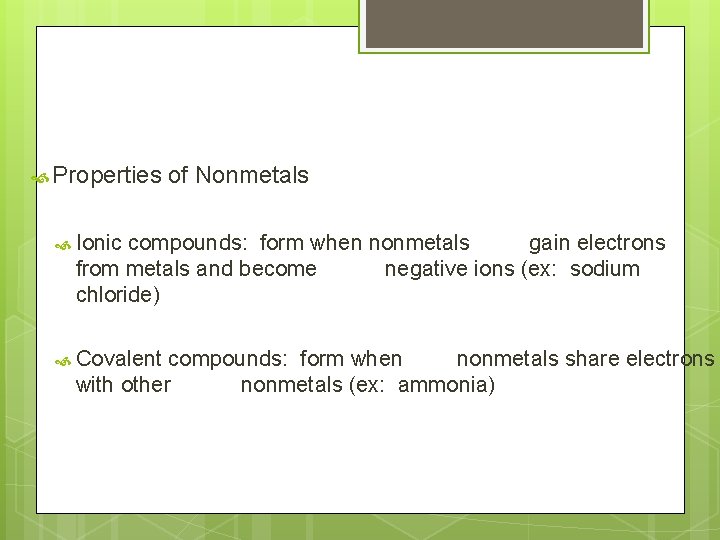
Properties of Nonmetals Ionic compounds: form when nonmetals gain electrons from metals and become negative ions (ex: sodium chloride) Covalent compounds: form when nonmetals share electrons with other nonmetals (ex: ammonia)

Hydrogen The most common element in the universe Mainly found as a diatomic molecule: two atoms of the same element in a covalent bond Highly reactive element found mostly on earth as part of a water compound

The Halogens Bromine, iodine, flourine, chlorine, astatine A salt forms when a halogen gains one electron from a metal Use of halogens Chlorine: disinfectant and bleach Bromine: dyes in cosmetics Iodine: hormone regulation

The Halogens Sublimation A solid changes directly into a gas without first becoming a liquid Iodine sublimates when heated

The Noble Gases Stable because their outer energy level react with other elements. Helium: Neon, used in blimps and balloons argon, and krypton: used in lights Neon, argon – neon lights Argon, krypton – electric lights and lasers is full; they do not

The Properties of Metalloids Form ionic & covalent bonds Have some metallic & some non-metallic properties Partial conduction gives them semiconductor characteristics

The Boron Group Named Boron: for the first element in Group 13 used in water softening products, antiseptics, and fuels Aluminum: abundant in earth’s crust; used in cans, foil, pans, building materials, and aircraft

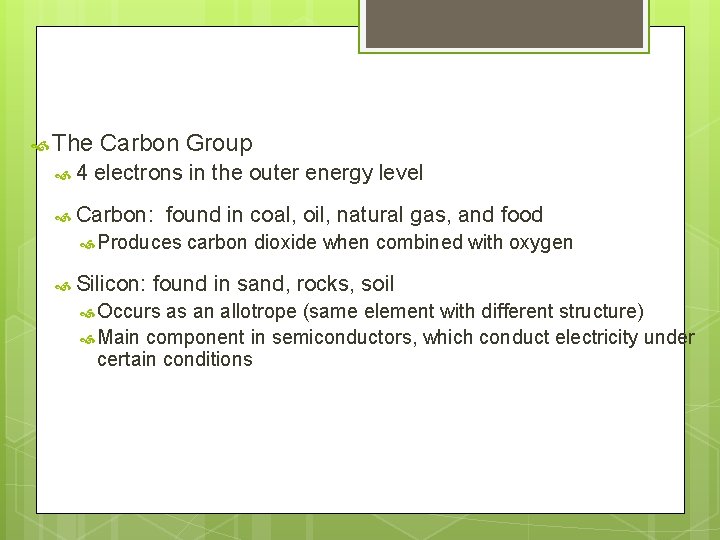
The 4 Carbon Group electrons in the outer energy level Carbon: found in coal, oil, natural gas, and food Produces Silicon: carbon dioxide when combined with oxygen found in sand, rocks, soil Occurs as an allotrope (same element with different structure) Main component in semiconductors, which conduct electricity under certain conditions

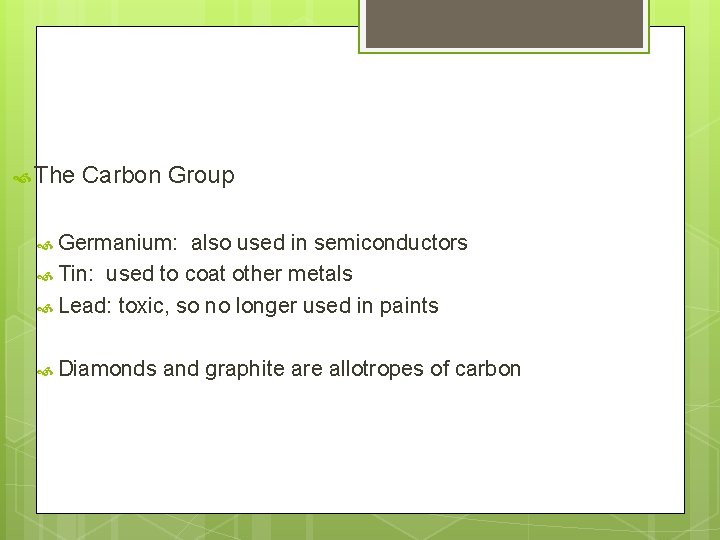
The Carbon Group Germanium: also used in semiconductors Tin: used to coat other metals Lead: toxic, so no longer used in paints Diamonds and graphite are allotropes of carbon

The Nitrogen Group 5 electrons in the valence shell Tend to form covalent bonds Nitrogen: used to make nitrates & ammonia Phosphorous: used in water softeners, fertilizers, match heads, fine china Antimony & bismuth: used with other metals to lower their melting points

The Oxygen Group 16 Oxygen: makes up 20% of air, used by living things in respiration, provides protection from the sun’s radiation in the form of ozone Sulfur: used to form sulfides in paint pigment Selenium: used in photocopiers & multivitamins Tellurium & polonium: also oxygen elements

Synthetic Elements Synthetic means “man-made” Scientists create elements not usually found on earth They usually disintegrate quickly

Synthetic Elements Uranium can be made into neptunium which forms plutonium when it disintigrates Plutonium can be changed into americium, which is used in smoke detectors

Synthetic Elements Transuranium elements have more than 92 protons and are synthetic & unstable Studying these elements help scientists understand the forces holding the nucleus together Element 114 lasted 30 seconds It combined 114 protons with 175 neutrons It broke apart due to the enormous repulsion between the protons