Elements Forming Compounds Chapter 10 Lesson 2 Page










- Slides: 18

Elements Forming Compounds Chapter 10 Lesson 2 Page 364

How Do Ionic Compounds Form • When a neutral atom transfers one or more electrons to another atom, it results in the formation of an ionic compound.

Ions • Ion- an atom or group of atoms that has an electric charge by losing or gaining electrons • Metal atoms tend to lose electrons • Nonmetals tend to gain electrons • The objective is to gain/lose enough electrons to become “stable” or have a full valence shell

Common Ions • Look at the chart on page 366 • Ions can be made of several atoms • Ions that are made of more than one atom are called polyatomic ions • Polyatomic ions will have an overall positive or negative charge

Ionic Bonds • When atoms that easily lose/gain electrons they transfer the electrons from one type of atom to the other • The transfer make each atom more stable • Read page 366 at the bottom • Ionic bond- oppositely charged particles attract a bond • Ionic compound- made of positive and negative ions, but the overall charge is zero

Formulas of Ionic Compounds • Chemical formula- group of symbols that shows the ratio of elements in a compound • Atoms will combine to form a compound with zero charge • Look at page 367 • Subscript- the small number at the bottom of the letters, tells the ratio of elements in a compound, is there is no number then it means 1.

Naming Ionic Compounds • Ionic compound names start with the positive ion first, followed by the negative ion • Read page 368 to figure out where names for different compounds came from. • You will get to name compounds in high school chemistry

How Do Molecular Compounds Form? • Covalent Bond- formed when atoms share electrons, normally formed between nonmetals • Covalent bonds between the shared electrons of each atom hold the atoms together to form a molecular compound

Electron Sharing • Molecular compound- compound that is made up of molecules • Molecules- a neutral group of atoms joined together by covalent bonds

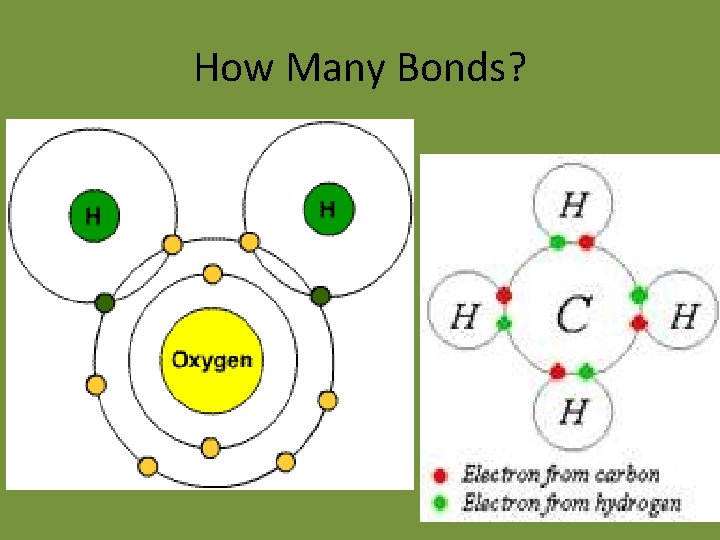
How Many Bonds?

Nonpolar and Polar Bonds • Electrons move to different atoms like in game of tug-of-war in covalent bonds • Some atoms have a stronger pull than others in a covalent bond • Unequal sharing causes covalent bonds to have a slight charge (weaker than ion)

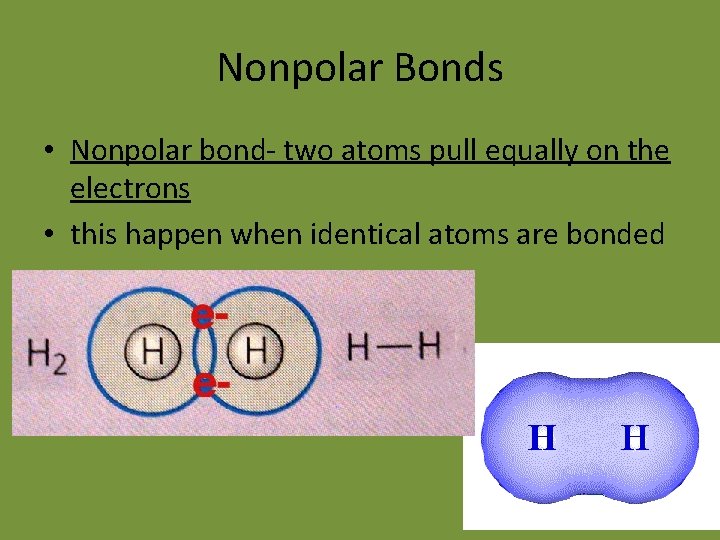
Nonpolar Bonds • Nonpolar bond- two atoms pull equally on the electrons • this happen when identical atoms are bonded

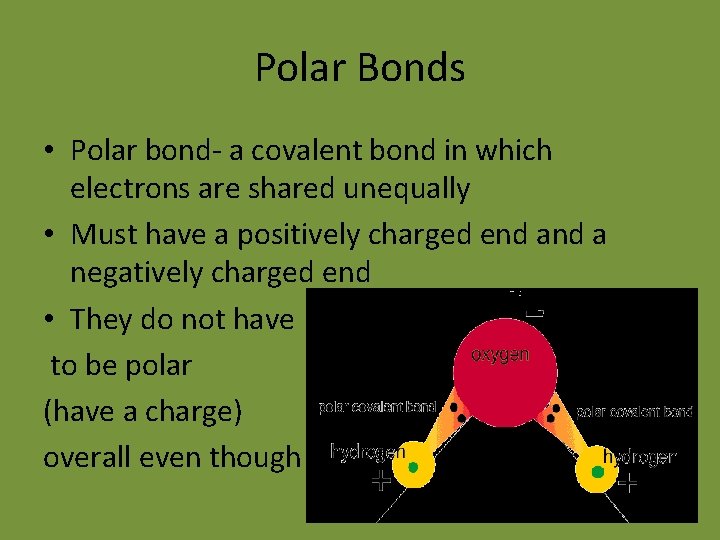
Polar Bonds • Polar bond- a covalent bond in which electrons are shared unequally • Must have a positively charged end a negatively charged end • They do not have to be polar (have a charge) overall even though some do

Attractions Between Molecules • Opposites attract!!!! • Molecules are connected by their weak attraction of the slight negative and slight positive charges • These attractions are called intermolecular forces

Attraction Between Molecules • For example: the polarized negative end of the oxygen atom in a water molecule attracts the polarized positive charge of hydrogen end of a nearby water molecule • This pulls water molecules together (intermolecular force) • Melting and boiling point differ based on the strengths/weakness of the different molecular bonds

Properties of Metals Properties include: Luster- shine Malleability-able to be rolled into thin sheets Ductility-ability to be stretched into wire Electrical conductivity- transfer electrical current • Thermal conductivity- transfer heat • • •

Properties of Metals • Each property of metals is determined by the structure of metal atoms and the bonding between their valence electrons • Metals usually lose VE

Classifying Chemical Compounds Chapter 10 Lesson 3 Page 374