Elements Compounds Mixtures EQ How can a substance

Elements, Compounds & Mixtures EQ: How can a substance be identified as an element, compound or mixture?
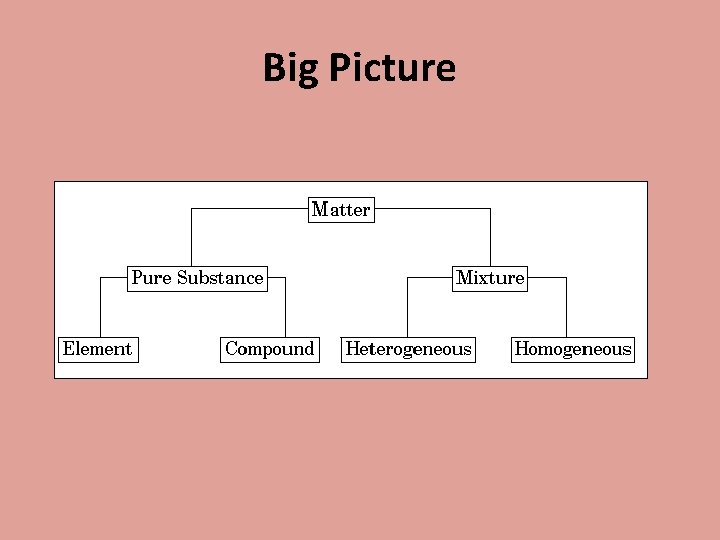
Big Picture
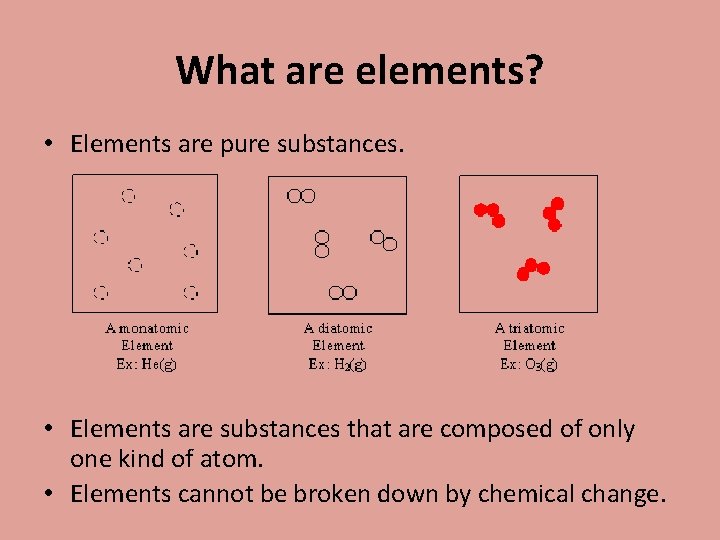
What are elements? • Elements are pure substances. • Elements are substances that are composed of only one kind of atom. • Elements cannot be broken down by chemical change.
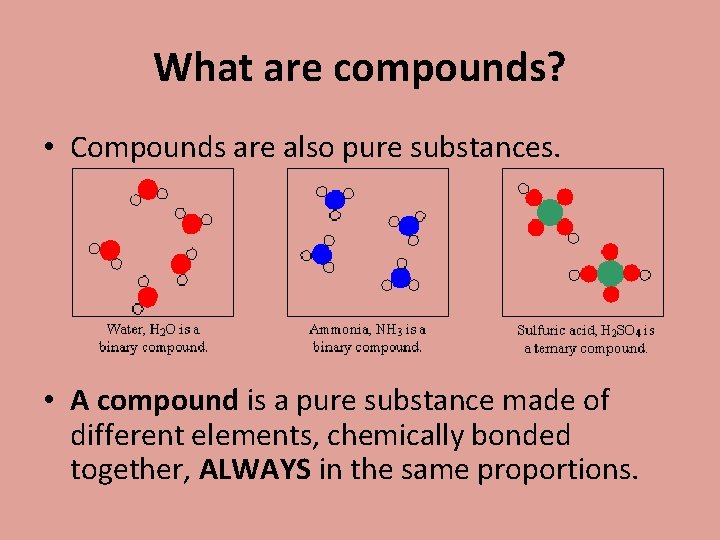
What are compounds? • Compounds are also pure substances. • A compound is a pure substance made of different elements, chemically bonded together, ALWAYS in the same proportions.
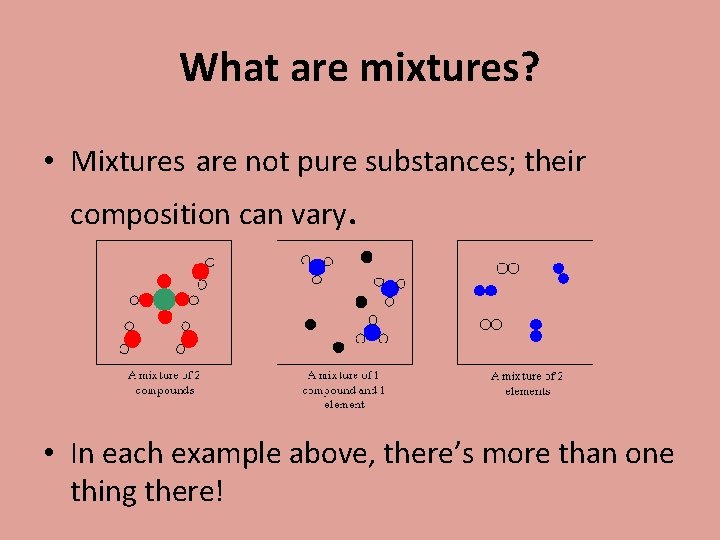
What are mixtures? • Mixtures are not pure substances; their composition can vary. • In each example above, there’s more than one thing there!
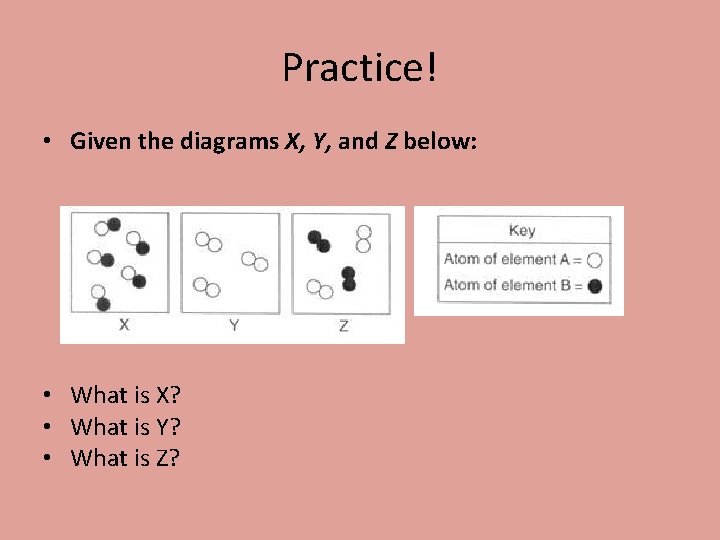
Practice! • Given the diagrams X, Y, and Z below: • What is X? • What is Y? • What is Z?
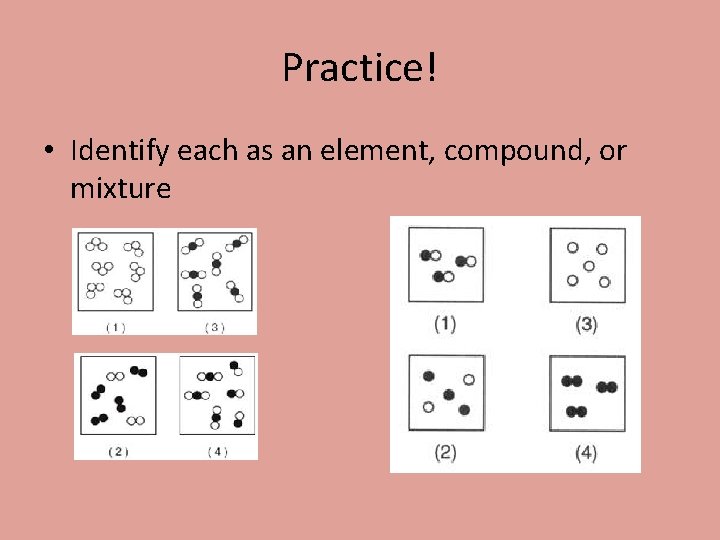
Practice! • Identify each as an element, compound, or mixture
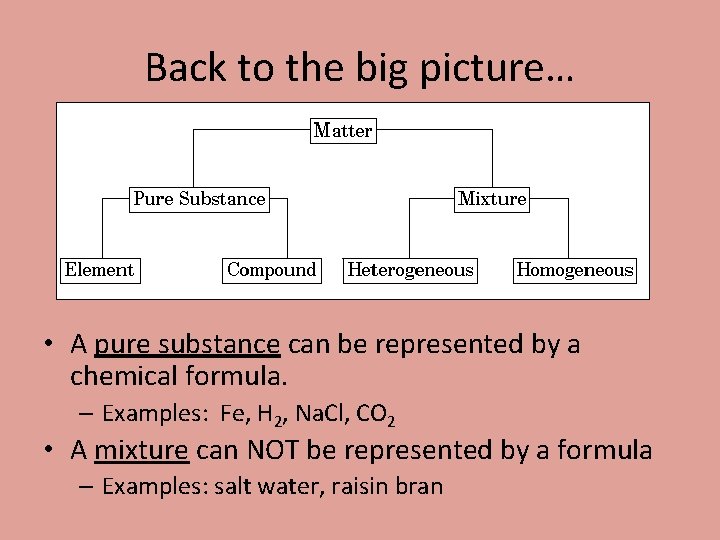
Back to the big picture… • A pure substance can be represented by a chemical formula. – Examples: Fe, H 2, Na. Cl, CO 2 • A mixture can NOT be represented by a formula – Examples: salt water, raisin bran

What are the 2 types of mixtures? • Heterogeneous Mixtures – Mixture that does not have the same properties throughout the mixture – Individual substances remain distinct – “hetero” = different • Homogeneous Mixtures – Mixture that has the same properties throughout. – A solution is a homogenous mixture – “homo” = same

Practice! • Identify the following a heterogeneous or homogeneous mixtures.
- Slides: 10