Elements Compounds Formulas Textbook Sections 14 5 Elements
Elements, Compounds, & Formulas Textbook Sections 14. 5

Elements �(def. ) Matter in its purest form �Made up of the same atoms

Compounds H 2 O �(def. ) A substance made up of 2 or more different elements �Chemically bonded

Properties of Compounds �Very different from the elements they are made of �Elements are chemically bonded which affects how they behave together
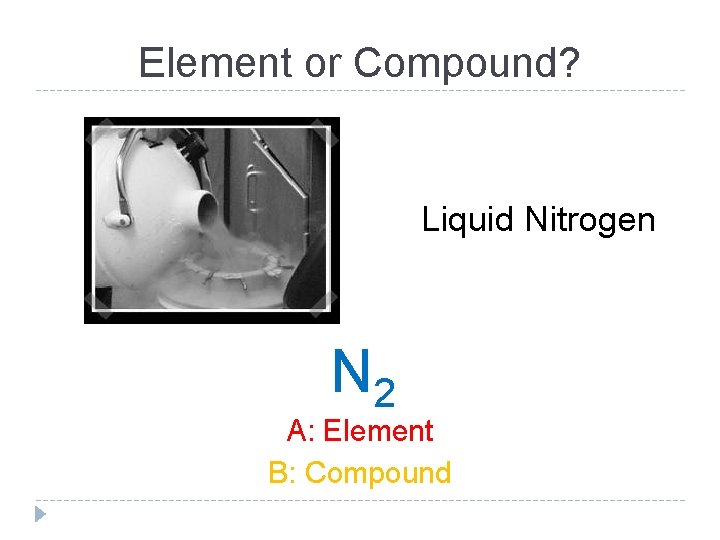
Element or Compound? Liquid Nitrogen N 2 A: Element B: Compound
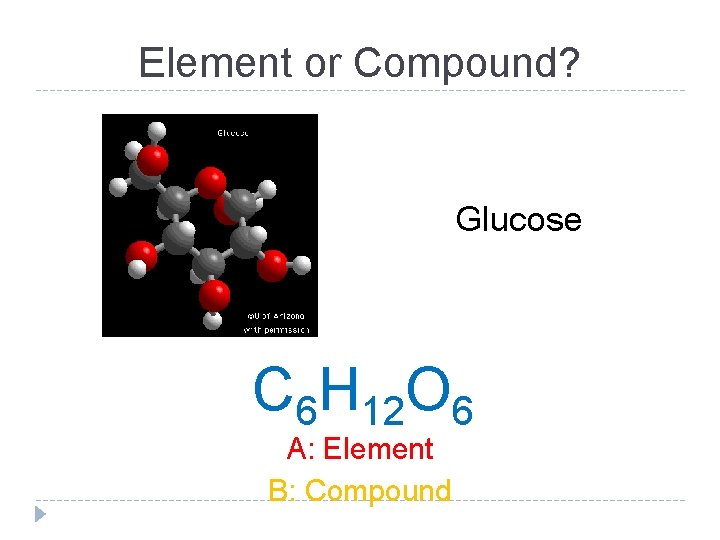
Element or Compound? Glucose C 6 H 12 O 6 A: Element B: Compound

Element or Compound? Hydrogen Peroxide H 2 O 2 A: Element B: Compound
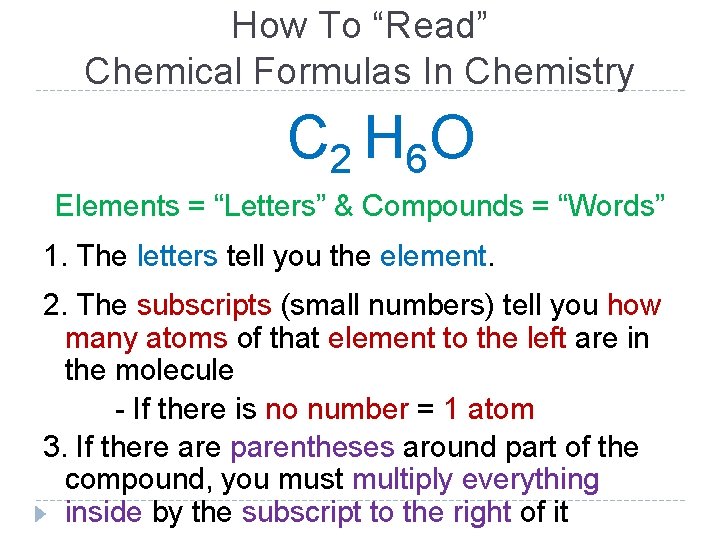
How To “Read” Chemical Formulas In Chemistry C 2 H 6 O Elements = “Letters” & Compounds = “Words” 1. The letters tell you the element. 2. The subscripts (small numbers) tell you how many atoms of that element to the left are in the molecule - If there is no number = 1 atom 3. If there are parentheses around part of the compound, you must multiply everything inside by the subscript to the right of it
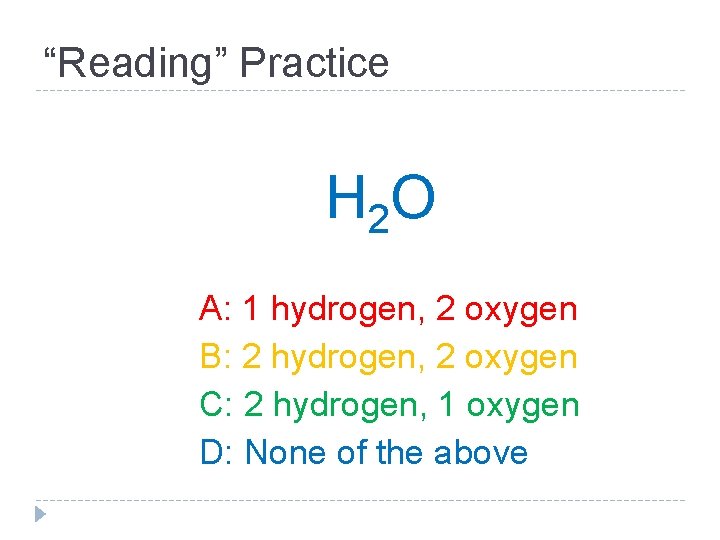
“Reading” Practice H 2 O A: 1 hydrogen, 2 oxygen B: 2 hydrogen, 2 oxygen C: 2 hydrogen, 1 oxygen D: None of the above
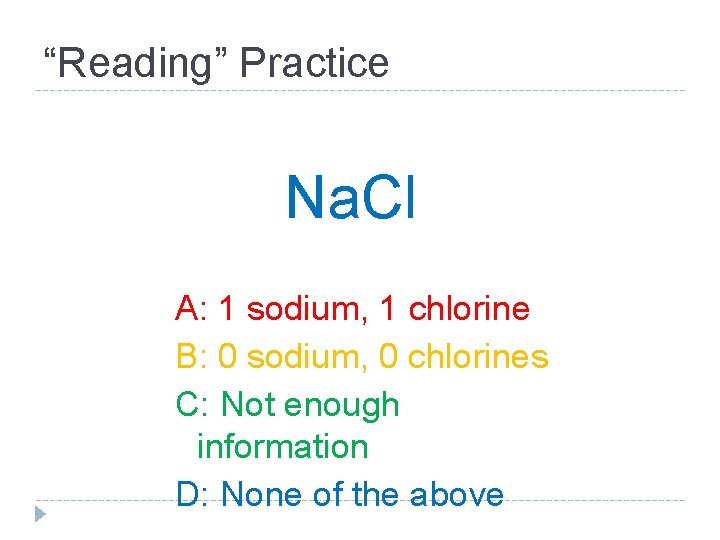
“Reading” Practice Na. Cl A: 1 sodium, 1 chlorine B: 0 sodium, 0 chlorines C: Not enough information D: None of the above
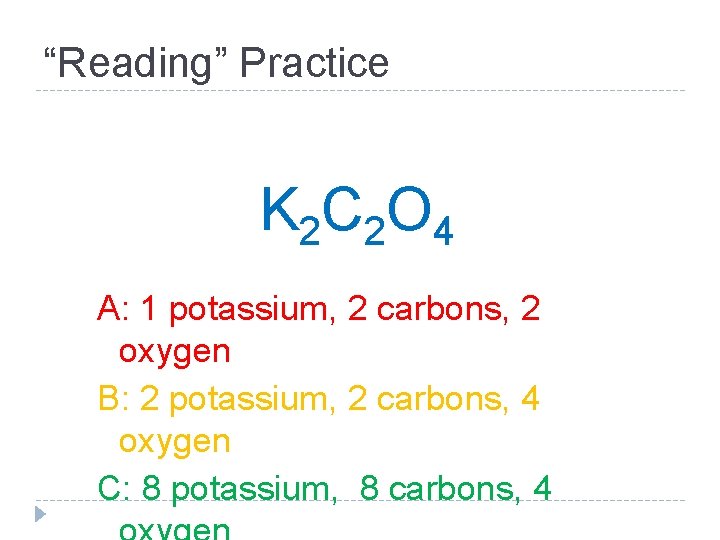
“Reading” Practice K 2 C 2 O 4 A: 1 potassium, 2 carbons, 2 oxygen B: 2 potassium, 2 carbons, 4 oxygen C: 8 potassium, 8 carbons, 4

“Reading” Practice Zr 3(PO 4)4 A: 1 zirconium, 3 phosphorus, 16 oxygen B: 3 zirconium, 1 phosphorus, 8 oxygen C: 3 zirconium, 4 phosphorus, 8 oxygen D: 3 zirconium, 4 phosphorus, 16 oxygen
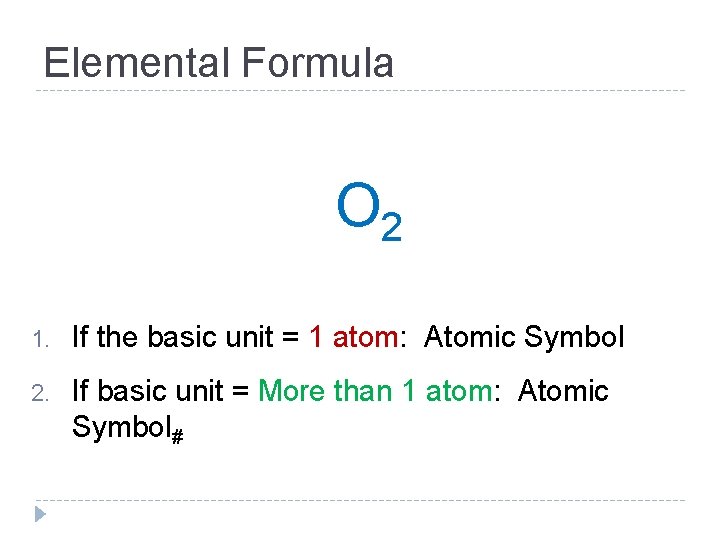
Elemental Formula O 2 1. If the basic unit = 1 atom: Atomic Symbol 2. If basic unit = More than 1 atom: Atomic Symbol#
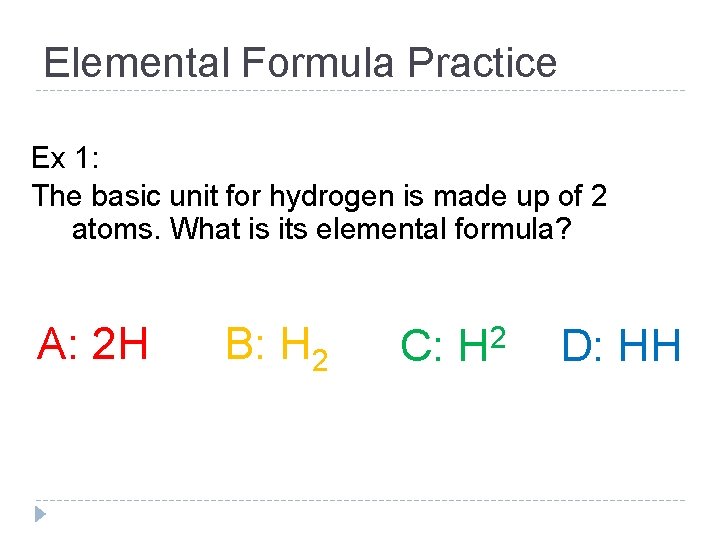
Elemental Formula Practice Ex 1: The basic unit for hydrogen is made up of 2 atoms. What is its elemental formula? A: 2 H B: H 2 C: 2 H D: HH
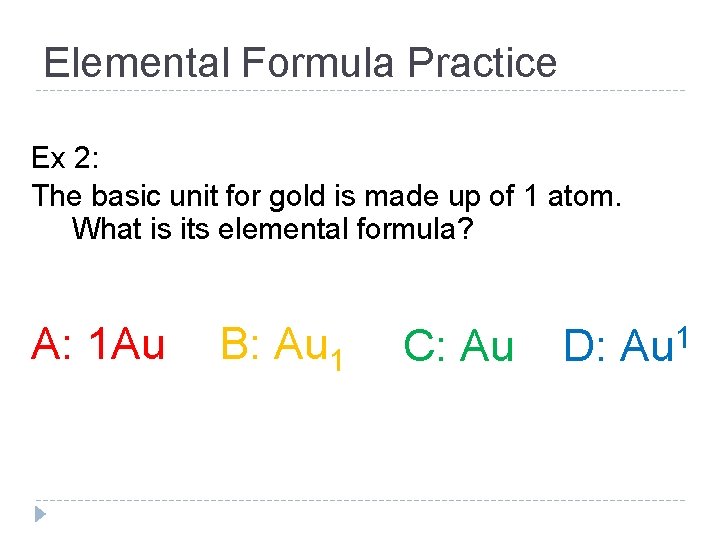
Elemental Formula Practice Ex 2: The basic unit for gold is made up of 1 atom. What is its elemental formula? A: 1 Au B: Au 1 C: Au D: 1 Au
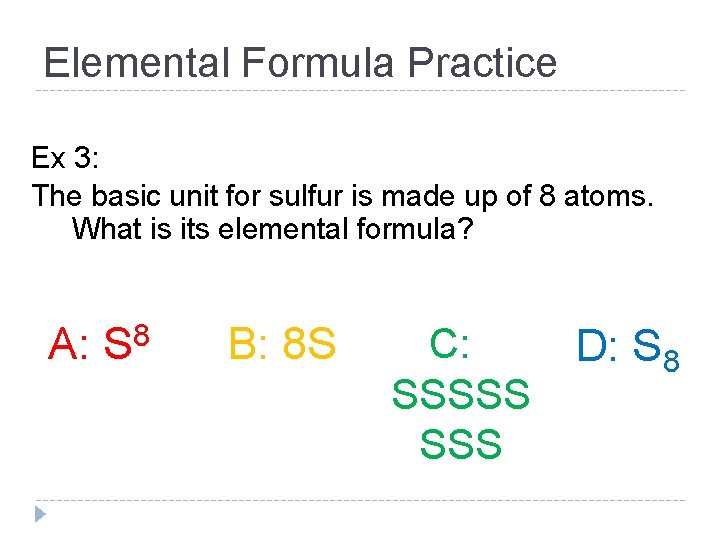
Elemental Formula Practice Ex 3: The basic unit for sulfur is made up of 8 atoms. What is its elemental formula? A: S 8 B: 8 S C: SSSSS D: S 8

In-Class Practice • Take out a piece of paper. • Write the questions AND your answers PART ONE: • With Your Partner, identify: • Whether the substance is a COMPOUND or ELEMENT • How many atoms of each ELEMENT is in the substance (write the element name!) READY?
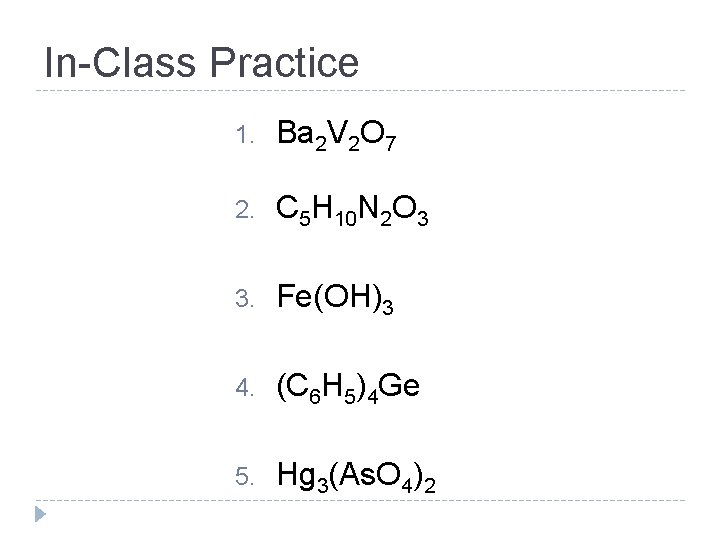
In-Class Practice 1. Ba 2 V 2 O 7 2. C 5 H 10 N 2 O 3 3. Fe(OH)3 4. (C 6 H 5)4 Ge 5. Hg 3(As. O 4)2

In-Class Practice PART TWO: • With Your Partner, identify: • Figure out the elemental formulas for the molecules described READY?
In-Class Practice 1. Chlorine can form a molecule by bonding 5 of its atoms together 2. The basic unit for sulfur is made from 2 sulfur atoms 3. Carbon can form a molecule made up of 20 of its atoms 4. What is the elemental formula if fluorine was able to create a molecule by bonding 3 of itself together?
- Slides: 20