Elements Compounds and Mixtures Chapter 4 Material on

































- Slides: 47

Elements, Compounds, and Mixtures Chapter 4 Material on Midterm

Mystery Mixture � What colors make up black ink?

Section 1: Elements �Objectives ◦ Describe pure substances ◦ Describe the characteristics of elements, and give examples ◦ Explain how elements can be identified ◦ Classify elements according to their properties

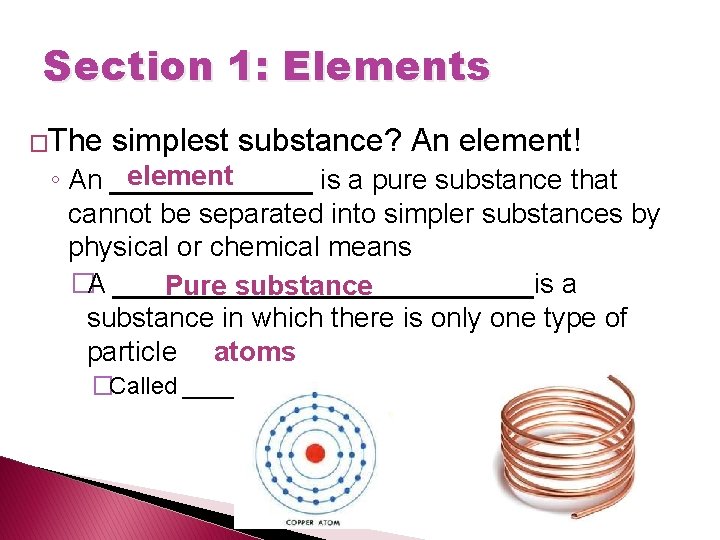
Section 1: Elements �The simplest substance? An element! element ◦ An _______ is a pure substance that cannot be separated into simpler substances by physical or chemical means �A ______________is a Pure substance in which there is only one type of particle atoms �Called ______

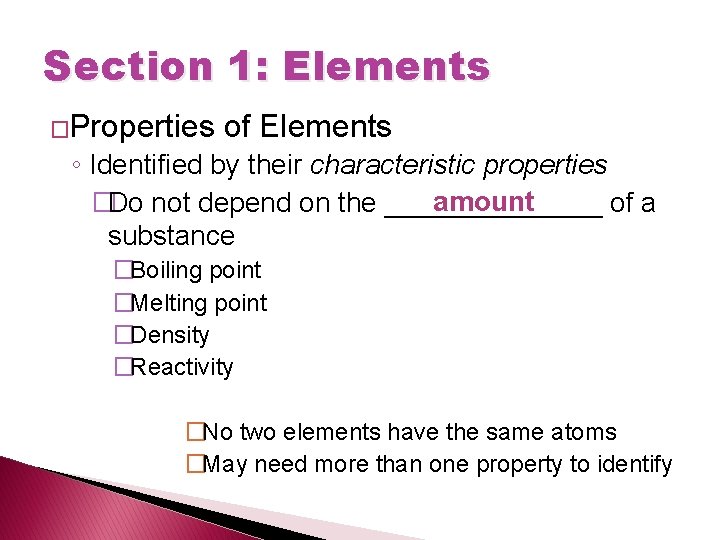
Section 1: Elements �Properties of Elements ◦ Identified by their characteristic properties amount �Do not depend on the _______ of a substance �Boiling point �Melting point �Density �Reactivity �No two elements have the same atoms �May need more than one property to identify

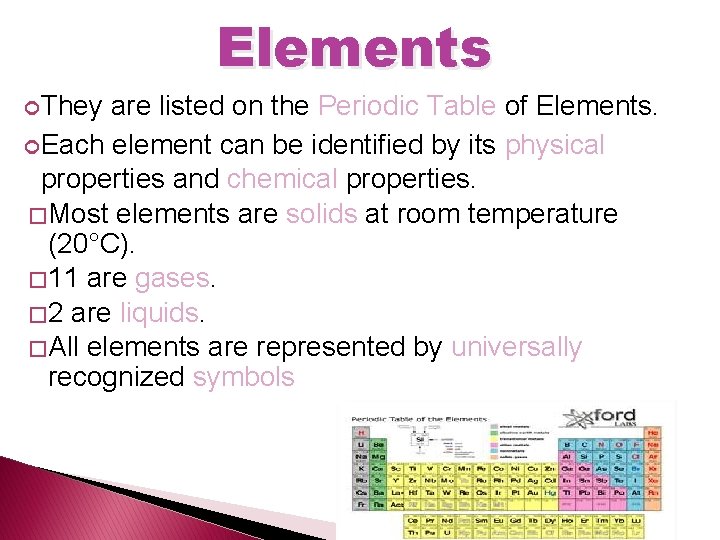
Elements They are listed on the Periodic Table of Elements. Each element can be identified by its physical properties and chemical properties. � Most elements are solids at room temperature (20°C). � 11 are gases. � 2 are liquids. � All elements are represented by universally recognized symbols

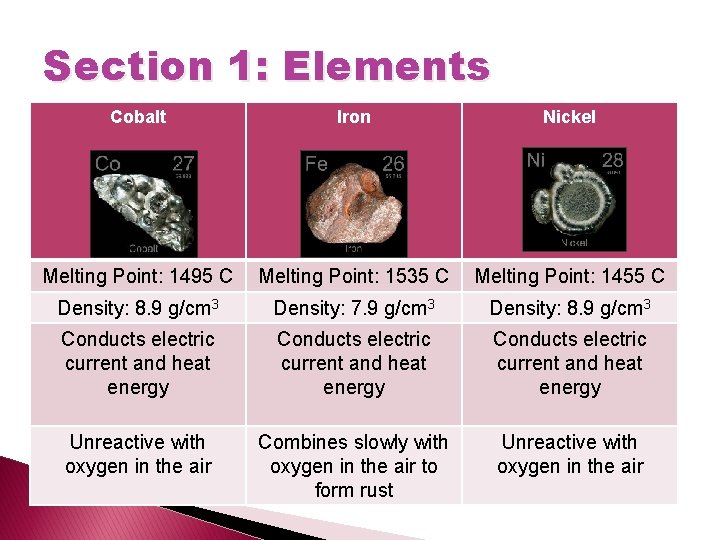
Section 1: Elements Cobalt Iron Nickel Melting Point: 1495 C Melting Point: 1535 C Melting Point: 1455 C Density: 8. 9 g/cm 3 Density: 7. 9 g/cm 3 Density: 8. 9 g/cm 3 Conducts electric current and heat energy Unreactive with oxygen in the air Combines slowly with oxygen in the air to form rust Unreactive with oxygen in the air

Classifying Elements Example: Dog Breeds How do you tell one breed from the other? Three categories of elements. Elements are organized on the Periodic Table by similar properties.

Section 1: Elements �Classifying Elements by their Properties ◦ Three main categories Metals �________ are elements that are shiny and good conductors of heat and electricity �_________ are elements that conduct Nonmetals heat and electricity poorly Metalloids �__________ are elements that have both properties of metals and nonmetals

Section 1: Elements

Section 1: Elements �Section Review ◦ Please answer the objectives on your summary sheet 1. Describe pure substances 2. Describe the characteristics of elements, and give examples 3. Explain how elements can be identified 4. Classify elements according to their properties

Section 2: Compounds �Objectives ◦ Explain how elements make up compounds ◦ Describe the properties of compounds ◦ Explain how a compound can be broken down into its elements ◦ Give examples of common compounds

Section 2: Compounds �What are compounds? ◦ A compound is a pure substance composed of two or more elements that are chemically combined _______ reaction �As a result of a _______

Compounds �Compounds – ◦ a substance made up of atoms of two or more elements chemically combined by chemical bonds. �When elements combine, they chemically react and undergo a chemical change.

Section 2: Compounds Compound Elements Combined Sodium chloride _________ Sodium and Chlorine Water _____ Hydrogen and Oxygen Vinegar Hydrogen, Carbon, and Oxygen Carbon dioxide ___________ Carbon and Oxygen Baking soda Sodium, Hydrogen, Carbon, and Oxygen

Section 2: Compounds �The Ratio of Elements in a compound ◦ A compound is a pure substance ◦ The ratios in a compound are ALWAYS THE SAME ____ H 2 O ◦ EX: Water is ______ �The water to oxygen mass has a ratio of 1: 8 �Same in 1 drop to 100 gallons NOT �Different ratio? _________! WATER

Section 2: Compounds �Properties of Compounds OWN physical ◦ Compounds have their _______ and chemical properties �EX: melting point, density, color �Physical or chemical? �EX: reactivity, flammability �Physical or chemical?

Section 2: Compounds �Properties Compounds versus Elements ◦ A compound will have different _______ than the elements that make properties it up

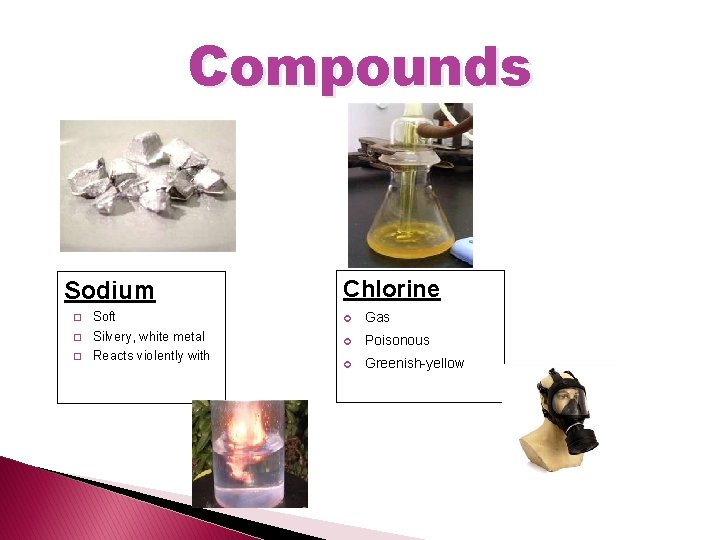
Compounds Sodium Chlorine � Soft Gas � Silvery, white metal Poisonous � Reacts violently with Greenish-yellow

But when chemically combined… You get……. . Na. Cl = Table salt!

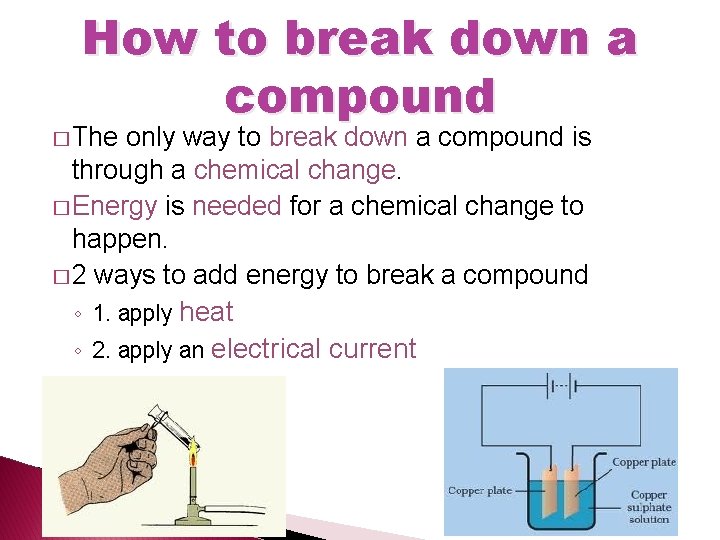
Section 2: Compounds �Breaking Down Compounds break down ◦ Compounds can ___________ into �Smaller compounds �Elements chemical ◦ Must be done by a ________ change �Add heat energy �Add electric energy

How to break down a compound � The only way to break down a compound is through a chemical change. � Energy is needed for a chemical change to happen. � 2 ways to add energy to break a compound ◦ 1. apply heat ◦ 2. apply an electrical current

Section 2: Compounds �Section Review ◦ Please answer the objectives on your summary sheet 1. Explain how elements make up compounds 2. Describe the properties of compounds 3. Explain how a compound can be broken down into its elements 4. Give examples of common compounds

Section 3: Mixtures � Objectives ◦ ◦ Describe three properties of mixtures Describe methods of separating the parts of a mixture Analyze a solution in terms of its solute and solvent Describe factors that affect solubility for solids, liquids, and gases ◦ Explain how concentration affects a solution ◦ Describe the particles in a solution, suspension, and colloid ◦ Explain the difference between colloids, solutions, and suspensions

Section 3: Mixtures �What is a mixture? ◦ A mixture is a combination of two or more substances that are not chemically combined ________________ �No chemical reaction �No compound formed �Each substance keeps its original identity _______ �Examples: pizza, sugar water, Italian dressing, sand, concrete

2 Categories of Mixtures Heterogeneous Mixture �a type of mixture in which the parts of the mixture are noticeably different from one another. ◦ Ex. Trail mix, pizza, Italian dressing Homogeneous mixture �a type of mixture in which the substances are so evenly distributed that it is difficult to distinguish one substance in the mixture from another. ◦ Ex. Sugar water, concrete

Section 3: Mixtures �Separating mixtures physical ◦ Use _______ means ◦ Can take more than one step

Section 3: Mixtures

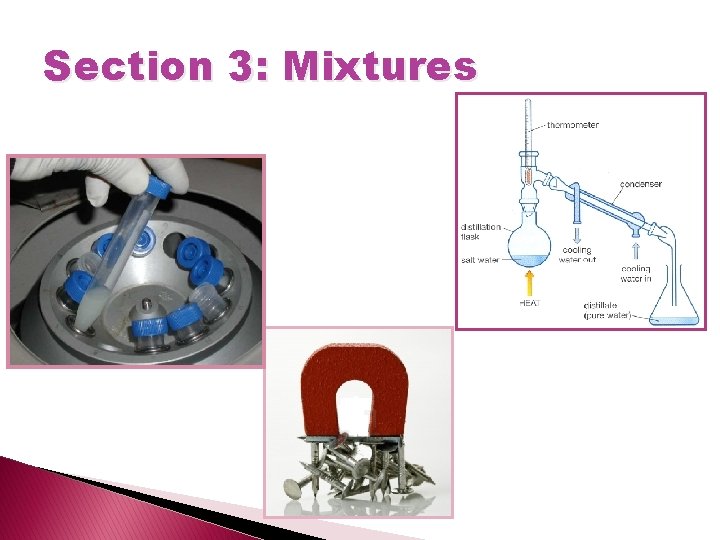
Separating a mixture � Distillation – a process that separates substances in a solution by using their boiling points. � Magnetism can be used to separate magnetic substances (Iron, Cobalt, and Nickel) from nonmagnetic substances.

Separating a mixture � Filtration - a process to separate materials based on their size. Ex: coffee filters and a screen to find artifacts at a historical site. �A Centrifuge can separate substances based of their densities. ◦ Denser materials separate first.

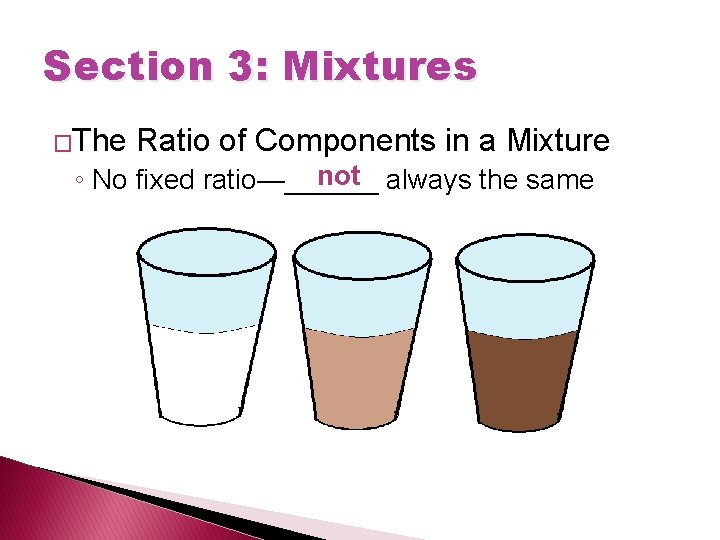
Section 3: Mixtures �The Ratio of Components in a Mixture not always the same ◦ No fixed ratio—______

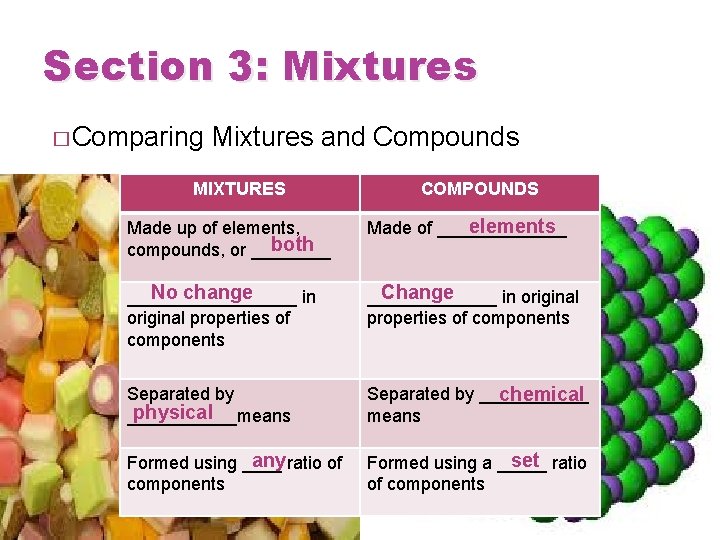
Section 3: Mixtures � Comparing Mixtures and Compounds MIXTURES COMPOUNDS Made up of elements, both compounds, or ____ elements Made of _______ No change _________ in original properties of components Change _______ in original properties of components Separated by physical ______means Separated by ______ chemical means any ratio of Formed using ____ components set ratio Formed using a _____ of components

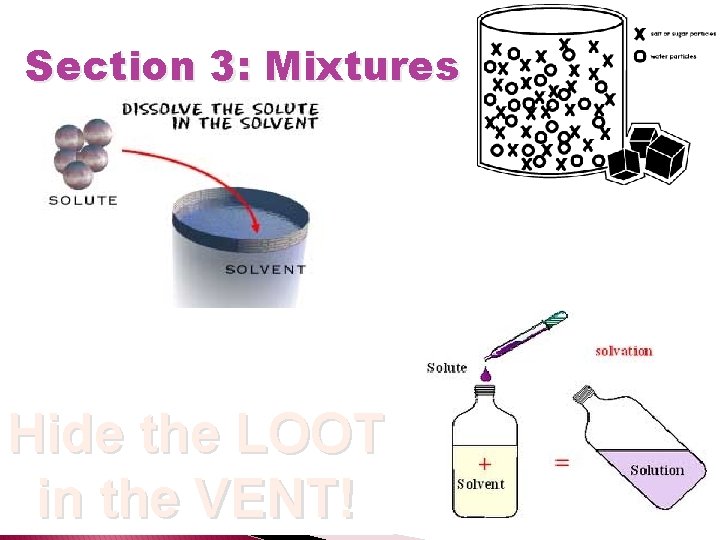
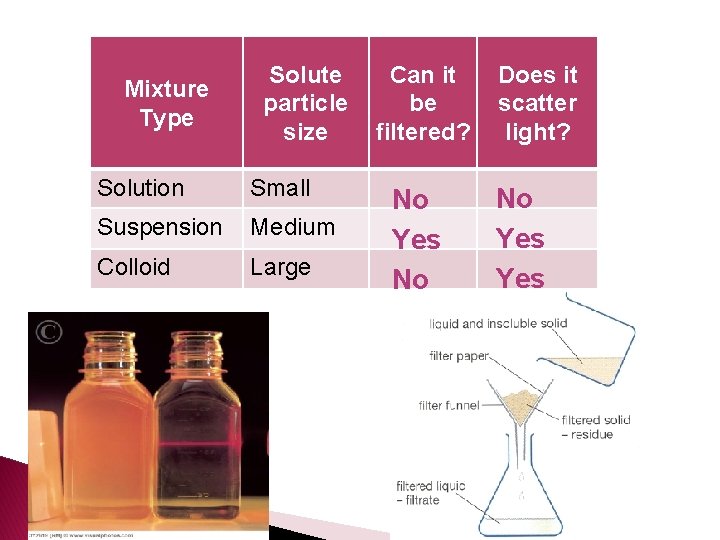
Section 3: Mixtures � What is a solution ◦ A solution is a mixture that appears to be a single substance Cannot �______ be filtered, cannot scatter light ◦ Who is who? solute �The _______ is the substance that is dissolved �The _______ is the substance in which the solute is solvent dissolved

Section 3: Mixtures Hide the LOOT in the VENT!

Section 3: Mixtures �Solutions form when the solute dissolves in the solvent does ◦ If it ____ dissolve, its soluble doesn’t ◦ If it _______ dissolve, it is insoluble

Section 3: Mixtures Solute State Solvent State Examples Gas Dry air (oxygen in nitrogen) Gas Liquid Soda (carbon dioxide in water) Liquid Antifreeze (alcohol in water) Solid Liquid Salt water (salt in water) Solid Brass (zinc in copper)

Section 3: Mixtures �Concentration of Solutions ◦ The concentration is a measure of the _____ of solute in a solvent amount �Units: g/m. L concentrated ◦ Lots of solute _________ dilute ◦ Little solute ________

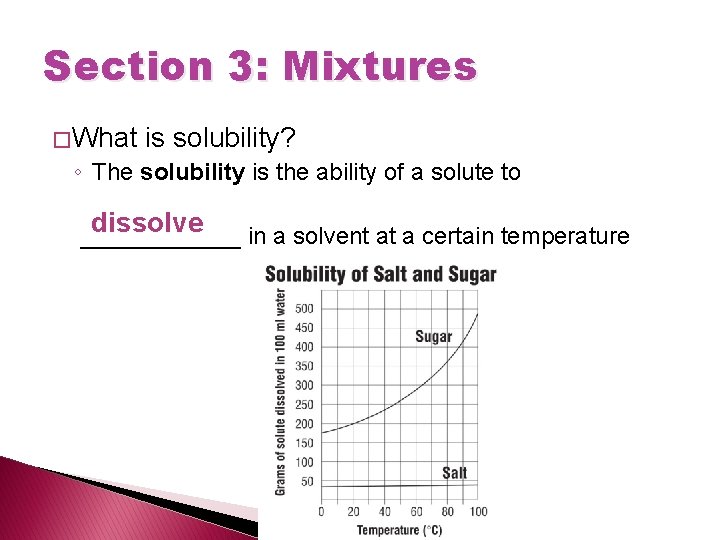
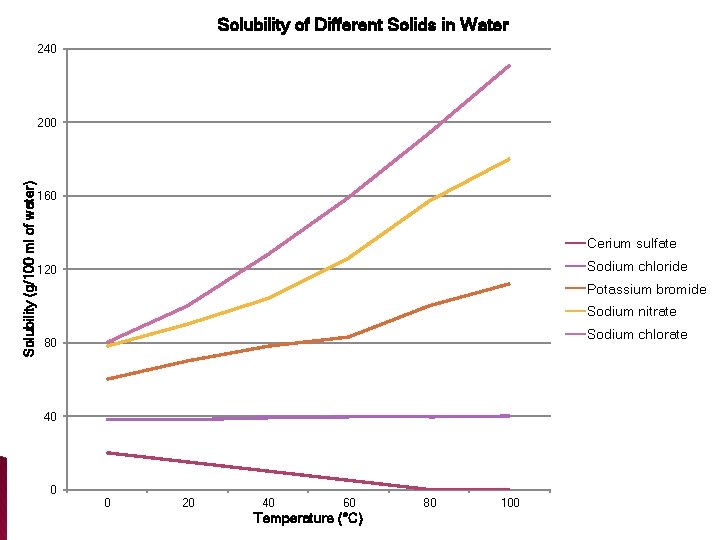
Section 3: Mixtures � What is solubility? ◦ The solubility is the ability of a solute to dissolve ______ in a solvent at a certain temperature

Solubility of Different Solids in Water 240 Solubility (g/100 ml of water) 200 160 Cerium sulfate Sodium chloride 120 Potassium bromide Sodium nitrate Sodium chlorate 80 40 0 0 20 40 60 Temperature (°C) 80 100

Section 3: Mixtures �How does temperature affect solubility? ◦ For liquid solvents less �A higher temperature makes a gas _______ soluble more �A higher temperature makes a solid _______ soluble �USUALLY �A higher temperature makes a liquid more soluble

Section 3: Mixtures ◦ To get solids to dissolve faster… Mix, stir, or shake ______________ - Causes particles to separate and spread out faster _______ Heat - Causes particles to move more quickly and separate ________ Crush - Spreads out solute to mix with solvent more quickly

Section 3: Mixtures �What is a suspension? ◦ A suspension is a mixture in which particles are large enough to be dispersed, but they settle out over time filtered � Can be ________, can scatter _____ light

Suspension A heterogeneous mixture that separates into layers after time. Need to be stirred or shaken. Ex. Italian dressing, snow globe, muddy water

Section 3: Mixtures �What is a colloid? ◦ A colloid is a mixture in which the particles are dispersed throughout but are not heavy enough to settle out � ______ be filtered, _______ Cannot can scatter light

Colloid - Form a homogeneous mixture. ◦ Ex. Milk, mayo, gelatin, whipped cream, stick of deodorant

Mixture Type Solute particle size Solution Small Suspension Medium Colloid Large Can it be filtered? No Yes No Does it scatter light? No Yes

Section 3: Mixtures � Section Review ◦ Please answer the objectives on your summary sheet 1. 2. 3. 4. Describe three properties of mixtures Describe methods of separating the parts of a mixture Analyze a solution in terms of its solute and solvent Describe factors that affect solubility for solids, liquids, and gases 5. Explain how concentration affects a solution 6. Describe the particles in a solution, suspension, and colloid 7. Explain the difference between colloids, solutions, and suspensions