Elements compounds and mixtures A mixture consists of

- Slides: 12

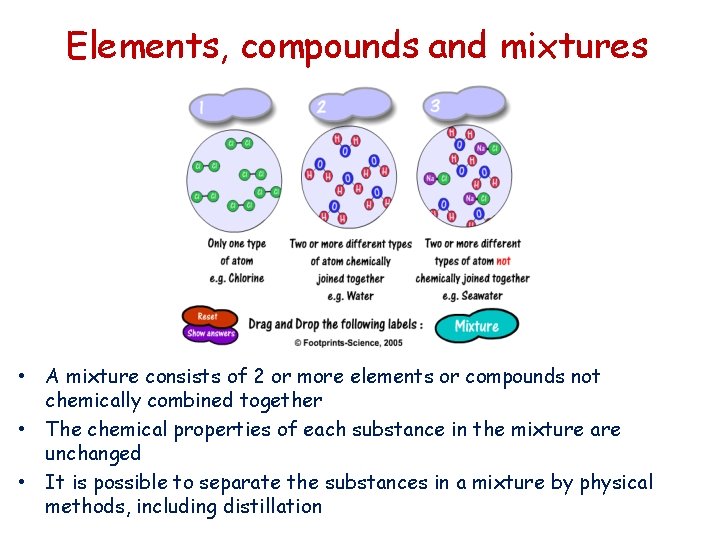
Elements, compounds and mixtures • A mixture consists of 2 or more elements or compounds not chemically combined together • The chemical properties of each substance in the mixture are unchanged • It is possible to separate the substances in a mixture by physical methods, including distillation

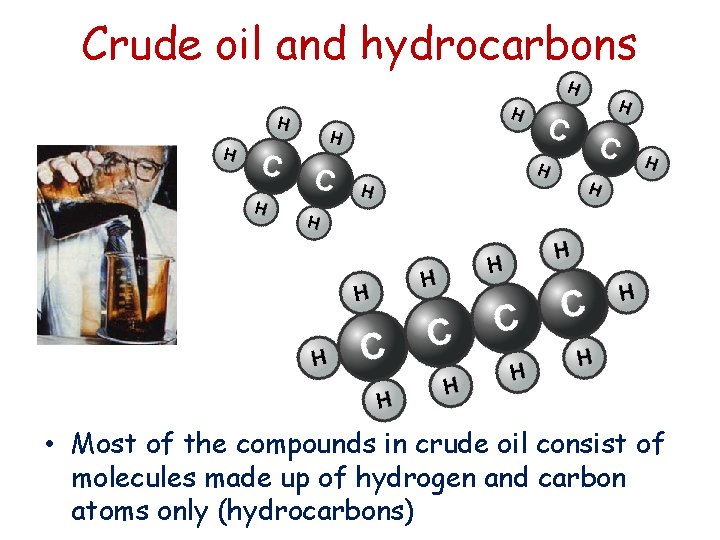
Crude oil and hydrocarbons • Most of the compounds in crude oil consist of molecules made up of hydrogen and carbon atoms only (hydrocarbons)

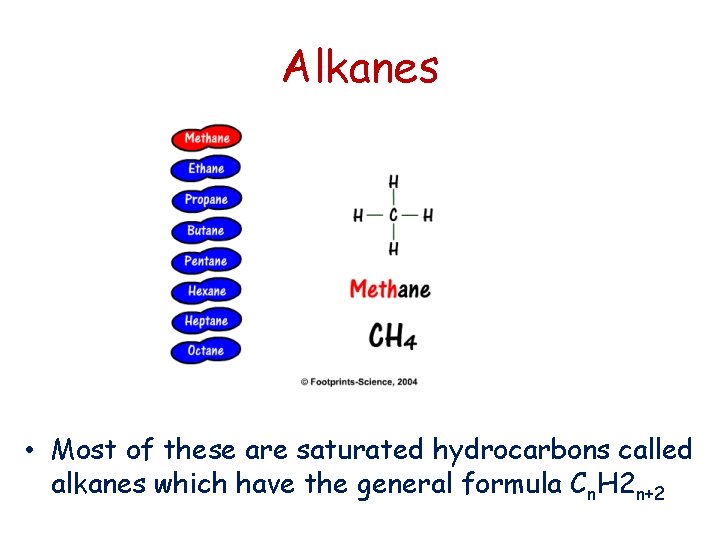
Alkanes • Most of these are saturated hydrocarbons called alkanes which have the general formula Cn. H 2 n+2

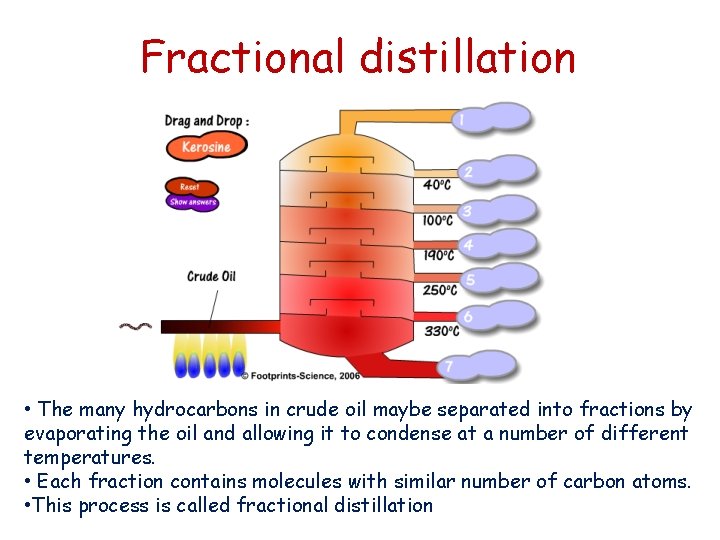
Fractional distillation • The many hydrocarbons in crude oil maybe separated into fractions by evaporating the oil and allowing it to condense at a number of different temperatures. • Each fraction contains molecules with similar number of carbon atoms. • This process is called fractional distillation

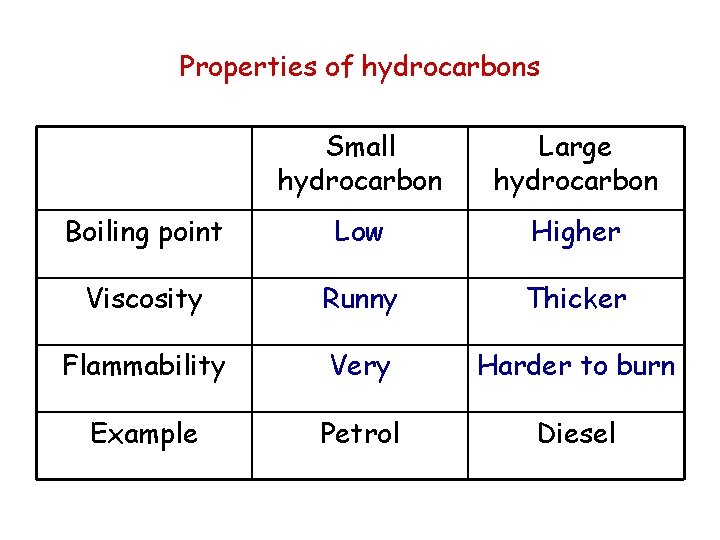
Properties of hydrocarbons Small hydrocarbon Large hydrocarbon Boiling point Low Higher Viscosity Runny Thicker Flammability Very Harder to burn Example Petrol Diesel

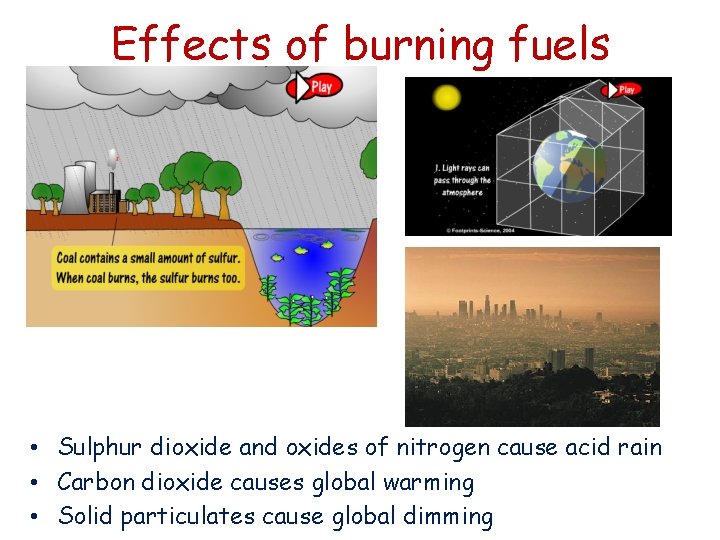
Burning fuels • The gases released into the atmosphere when a fuel burns may include carbon dioxide, water (vapour), carbon monoxide, sulphur dioxide, oxides of nitrogen and solid particles called particulates which may contain soot and unburnt fuels • The products of combustion relate to what elements were present in the fuel and whether the complete combustion took place

Effects of burning fuels • Sulphur dioxide and oxides of nitrogen cause acid rain • Carbon dioxide causes global warming • Solid particulates cause global dimming

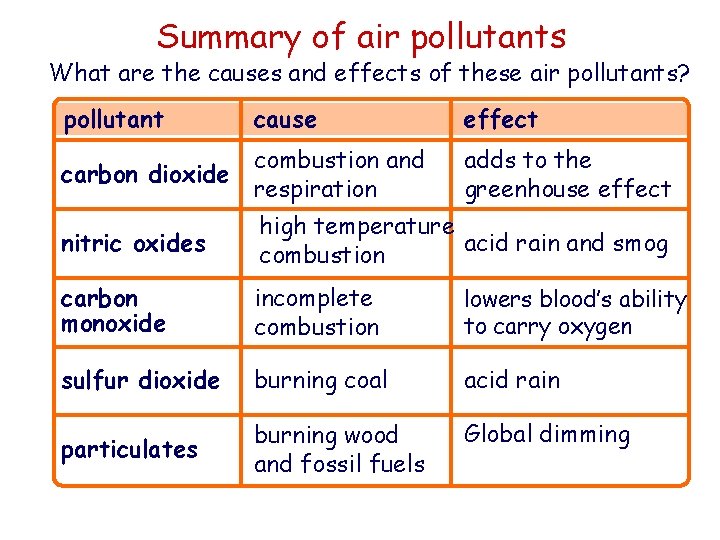
Summary of air pollutants What are the causes and effects of these air pollutants? pollutant cause combustion and carbon dioxide respiration effect adds to the greenhouse effect nitric oxides high temperature acid rain and smog combustion carbon monoxide incomplete combustion lowers blood’s ability to carry oxygen sulfur dioxide burning coal acid rain particulates burning wood and fossil fuels Global dimming

Reducing effects of burning fuels • Sulfur can be removed from fuels before they are burned • Sulfur dioxide can be removed from waste gases after combustion • There are economic, ethical and environmental reasons for using biofuels

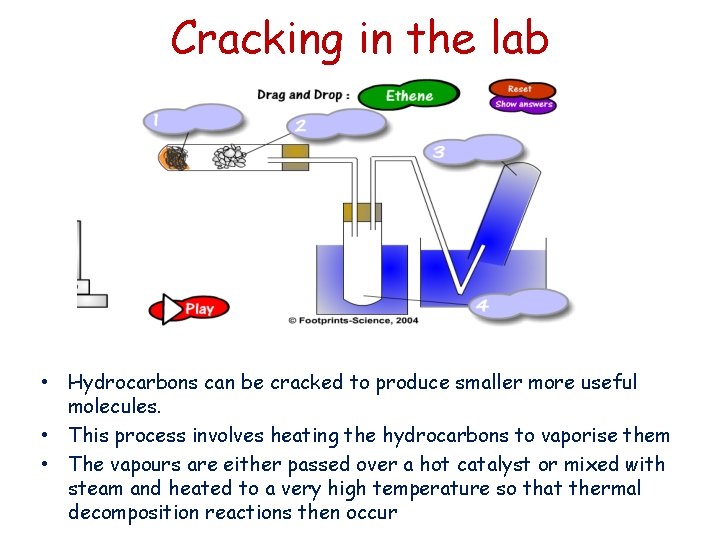
Cracking in the lab • Hydrocarbons can be cracked to produce smaller more useful molecules. • This process involves heating the hydrocarbons to vaporise them • The vapours are either passed over a hot catalyst or mixed with steam and heated to a very high temperature so that thermal decomposition reactions then occur

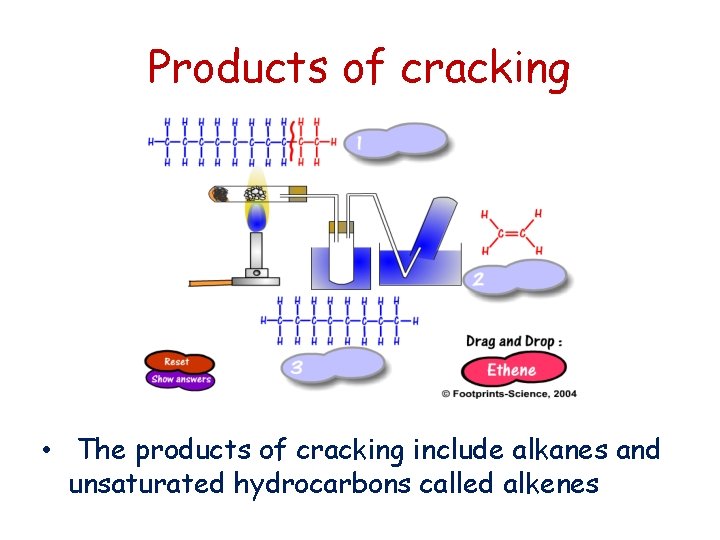
Products of cracking • The products of cracking include alkanes and unsaturated hydrocarbons called alkenes

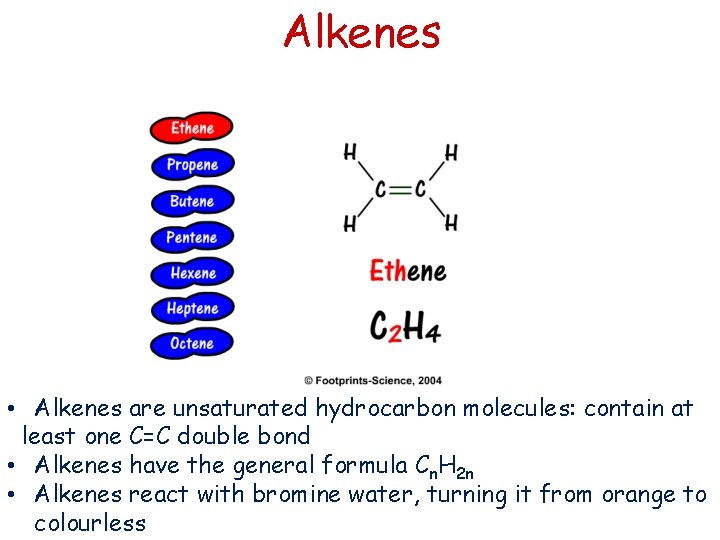
Alkenes • Alkenes are unsaturated hydrocarbon molecules: contain at least one C=C double bond • Alkenes have the general formula Cn. H 2 n • Alkenes react with bromine water, turning it from orange to colourless