Elements Atoms Particles The Periodic Table 4 1
Elements, Atoms, Particles & The Periodic Table

4. 1 All elements are composed of tiny indivisible particles called atoms.

4. 1 • Atoms of the same element are identical. The atoms of any one element are different from those of any other element.

4. 1 • Atoms of different elements can physically mix together or can chemically combine in simple ratios to form compounds.

Atomic Structure!

Protons • Positively Charged (+1) • Mass of 1 (amu) • Found in nucleus • # of protons = atomic number • Ex - Carbon always has 6 protons. Helium always has 2. Which element has 11 protons?

Neutrons • Neutral in Charge (no charge) • Mass of 1 (amu) • Found in nucleus • # of protons + neutrons = atomic mass • Number of neutrons can vary. Isotopes = atoms of the same element, that have different numbers of neutrons

12 6 C 14 6 C One isotope of Carbon is known as Carbon-14. Q: How many neutrons does Carbon-14 have? Atomic Mass - Atomic Number = # of neutrons (protons + neutrons)- (protons) = (neutrons)

Electrons • Negative Charge (-1) • Mass of 0 amu (has almost no mass) • Found in energy levels / shells • # of electrons = # of protons. • The first energy level can hold 2 electrons, later levels hold 8 Gold: Au How many electrons?

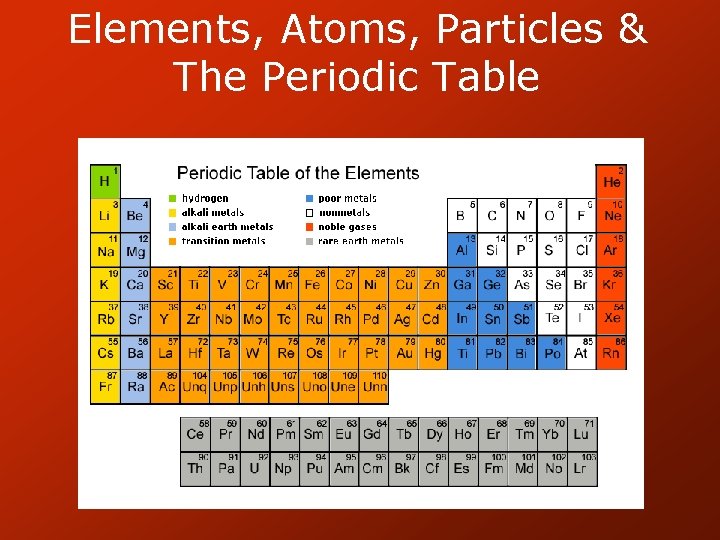
Periodic Table • Elements arranged by atomic number (number of protons) • Gives average atomic mass (most common isotope) • Can use to determine how many protons, neutrons and electrons each atom has.
- Slides: 10