Elements and The Periodic Table The Super 7
Elements and The Periodic Table
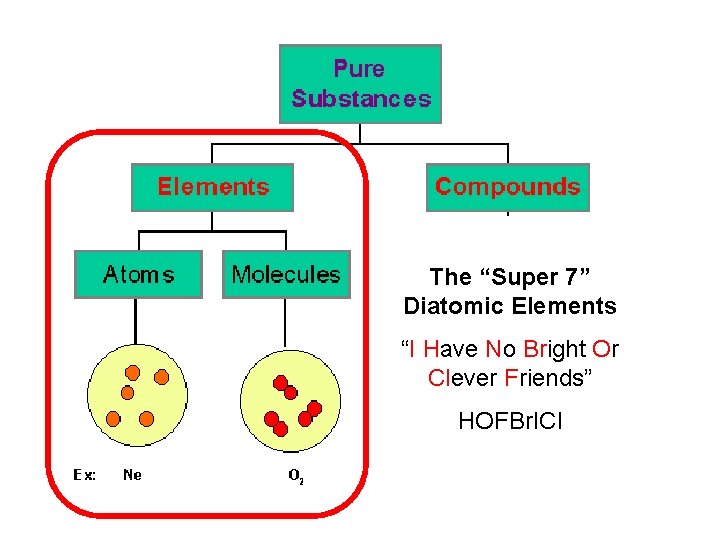
The “Super 7” Diatomic Elements “I Have No Bright Or Clever Friends” HOFBr. ICl
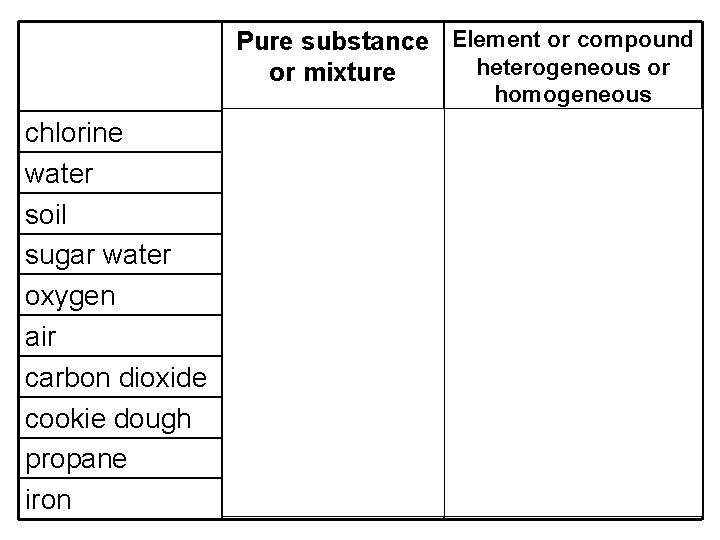
Pure substance Element or compound heterogeneous or or mixture homogeneous chlorine water soil sugar water oxygen air carbon dioxide cookie dough propane iron pure substance mixture pure substance element (molecule) compound heterogeneous homogeneous element (molecule) homogeneous compound heterogeneous compound element (atom)
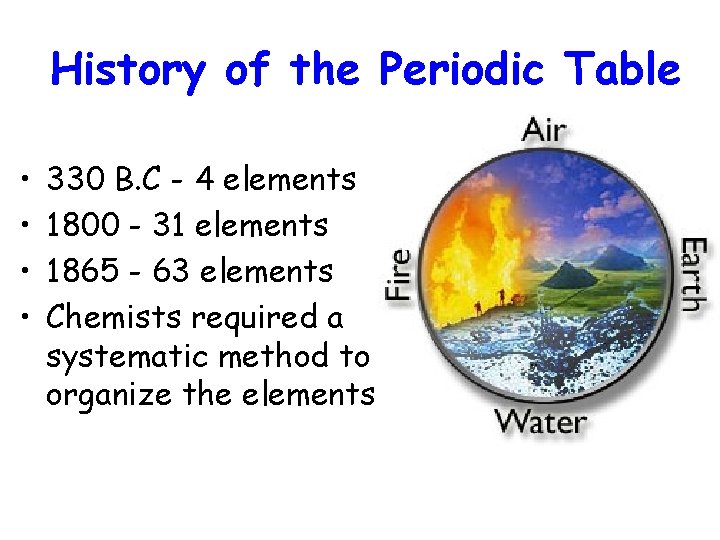
History of the Periodic Table • • 330 B. C - 4 elements 1800 - 31 elements 1865 - 63 elements Chemists required a systematic method to organize the elements

John Newlands • 1864 • Arranged all known elements in order of increasing atomic mass • Observed that every 8 th element had similar physical and chemical properties (Law of Octaves) • Began to group these elements into “families”

Lothar Meyer • 1865 • Also arranged the elements in order of increasing atomic mass • Found repeating patterns and developed a table of elements

Dimitri Mendeleev • 1869 • Noticed same patterns as Newlands & Meyer • Because Mendeleev published his table first, he is credited as the Father of the Periodic Table Now, elements are not arranged by atomic mass… Periodic Law: When arranged by atomic number, the properties of the elements repeat at regular intervals.

Amazing!

Within YOUR Lifetime
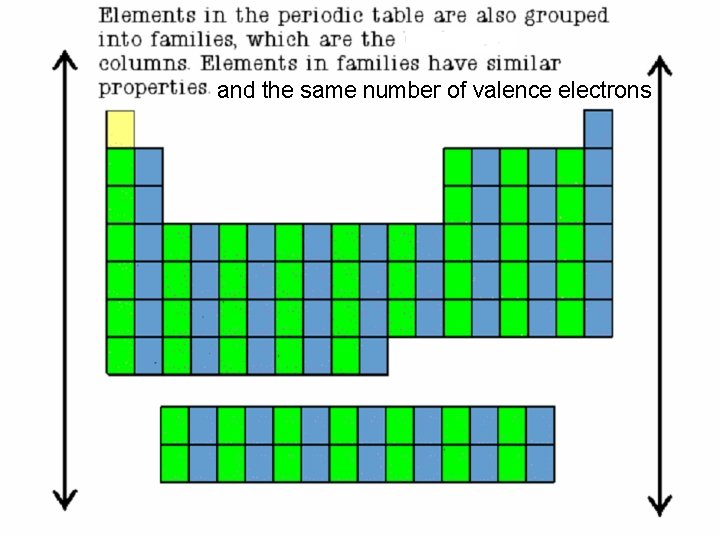
and the same number of valence electrons
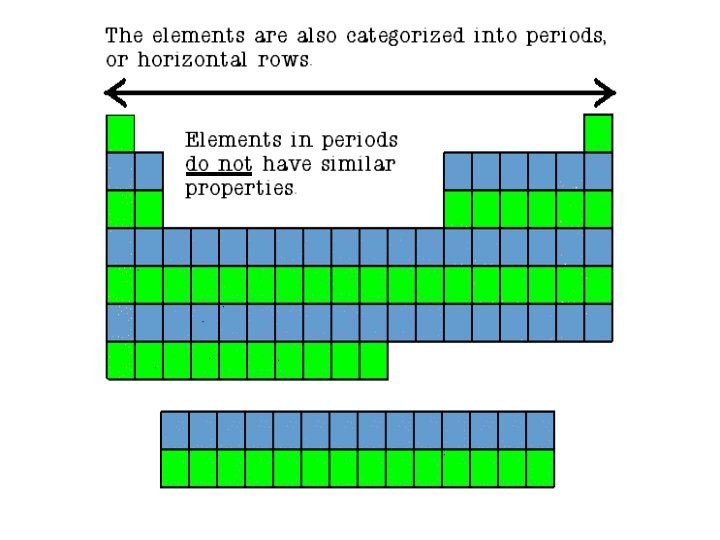

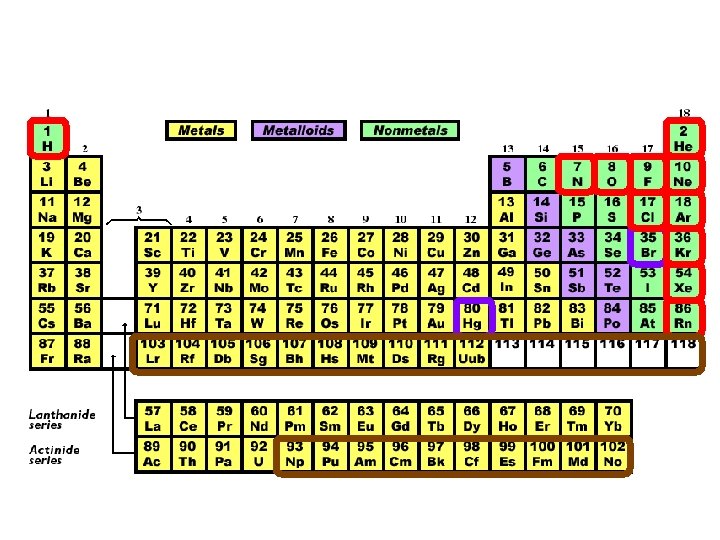
Hydrogen (A class of it’s own) • Sometimes it behaves like an alkali metal, sometimes like a halogen, and sometimes in its own unique way.
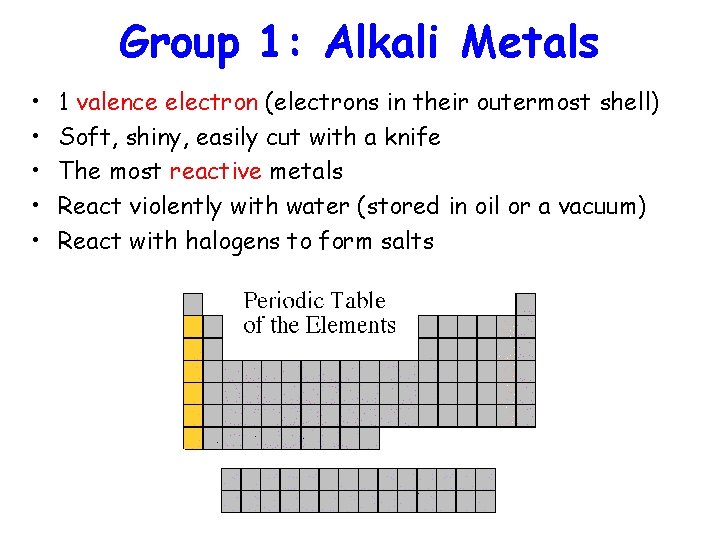
Group 1: Alkali Metals • • • 1 valence electron (electrons in their outermost shell) Soft, shiny, easily cut with a knife The most reactive metals React violently with water (stored in oil or a vacuum) React with halogens to form salts
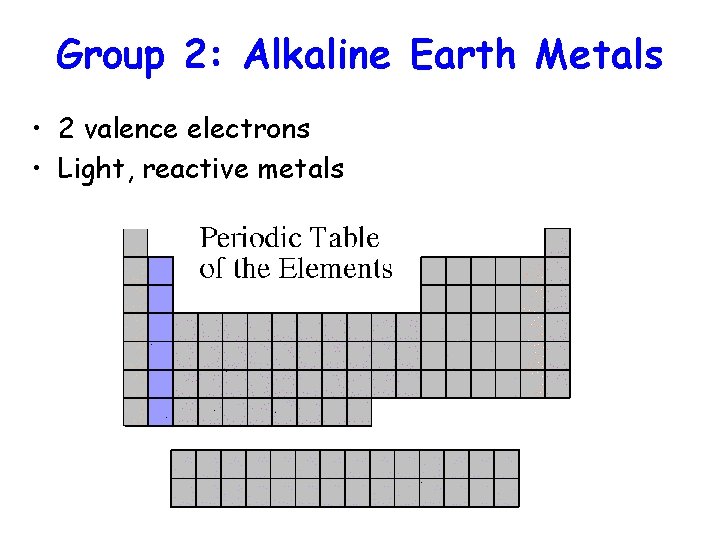
Group 2: Alkaline Earth Metals • 2 valence electrons • Light, reactive metals
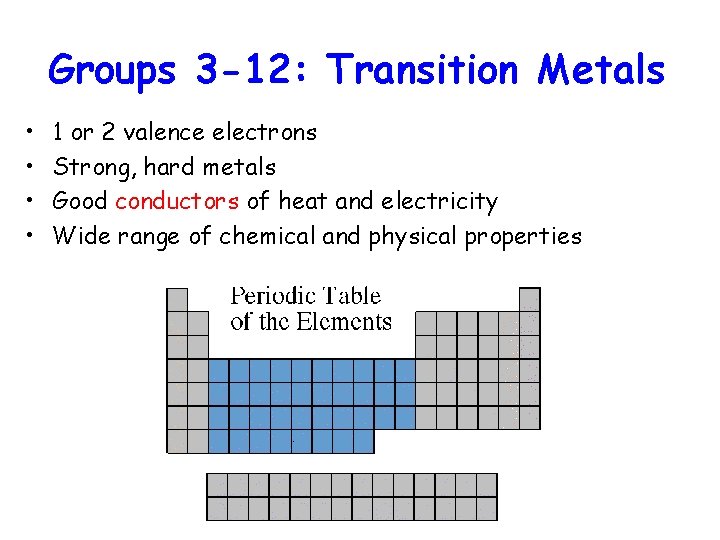
Groups 3 -12: Transition Metals • • 1 or 2 valence electrons Strong, hard metals Good conductors of heat and electricity Wide range of chemical and physical properties
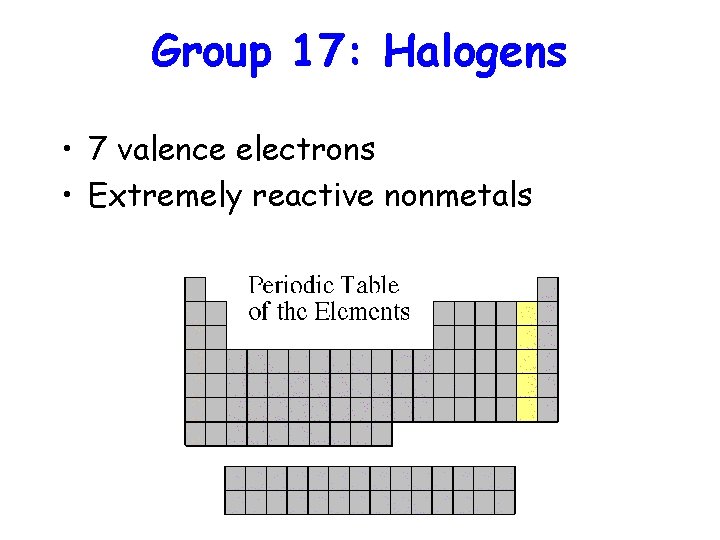
Group 17: Halogens • 7 valence electrons • Extremely reactive nonmetals
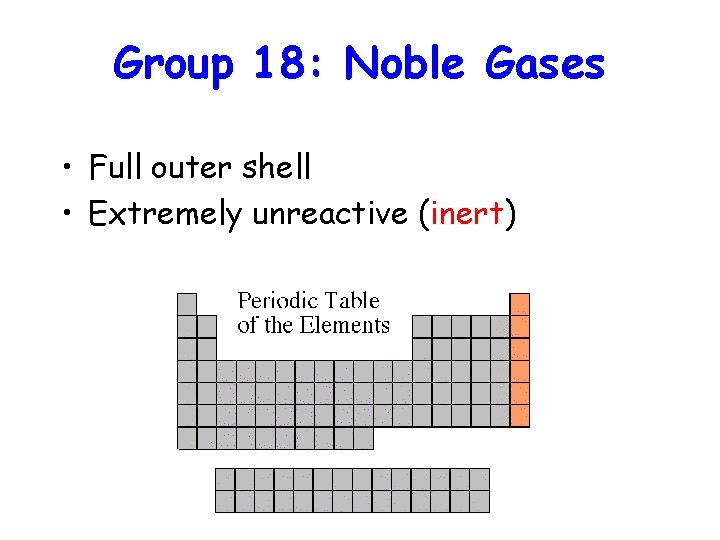
Group 18: Noble Gases • Full outer shell • Extremely unreactive (inert)
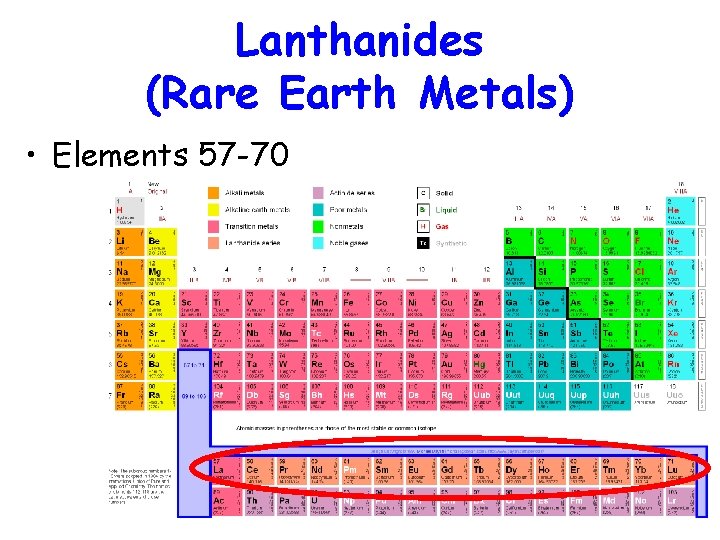
Lanthanides (Rare Earth Metals) • Elements 57 -70
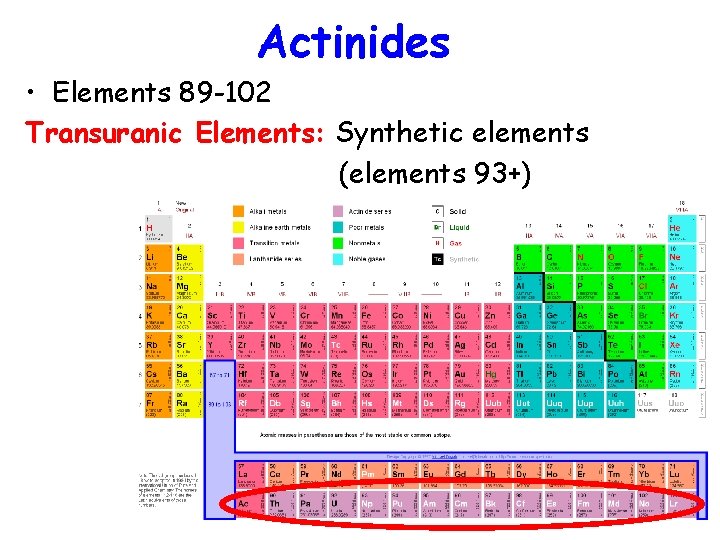
Actinides • Elements 89 -102 Transuranic Elements: Synthetic elements (elements 93+)

Practice! • p. 11 #1, 2 • p. 20 #16 -20 • p. 21 # 4
- Slides: 24