Elements and The Periodic Table Review What is

Elements and The Periodic Table

Review: What is an Element? An element is a substance that is made entirely from one type of atom.
What is the PERIODIC TABLE? Shows all known elements in the universe.

Rules for chemical symbols in the periodic table: 1. The symbol is usually the first one or two letters of the name. 2. Sometimes the Latin name is used. 3. The first letter of a symbol is always a capital letter. 4. The second letter of a symbol is always a small letter. 5. Every element has a different symbol.
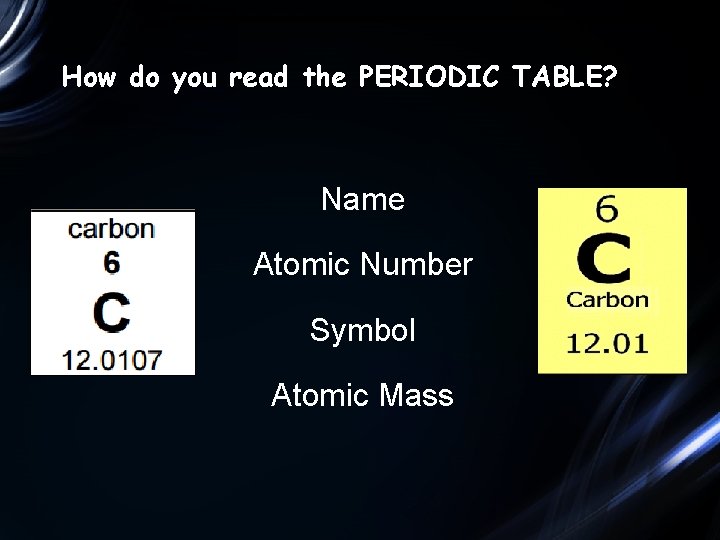
How do you read the PERIODIC TABLE? Name Atomic Number Symbol Atomic Mass
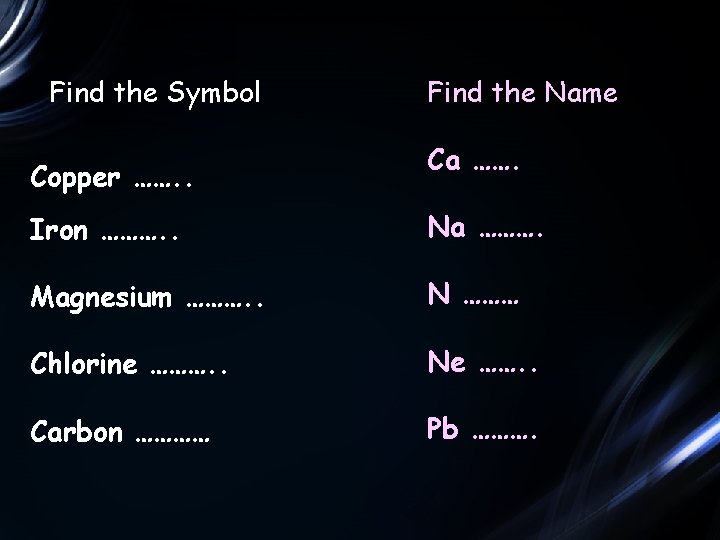
Find the Symbol Copper ……. . Find the Name Ca ……. Iron ………. . Na ………. Magnesium ………. . N ……… Chlorine ………. . Ne ……. . Carbon ………… Pb ……….
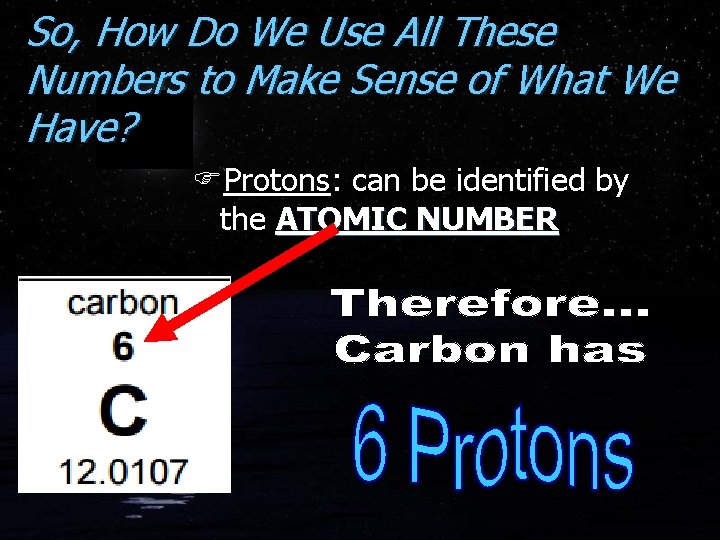
So, How Do We Use All These Numbers to Make Sense of What We Have? FProtons: can be identified by the ATOMIC NUMBER

So, How Do We Use All These Numbers to Make Sense of What We Have? FElectrons: can be identified by the ATOMIC NUMBER

So, Mrs. S… how can we find the Neutrons then? FNeutrons: can be found when taking the ATOMIC MASS and subtracting out the ATOMIC NUMBER ATOMIC MASS ATOMIC NUMBER
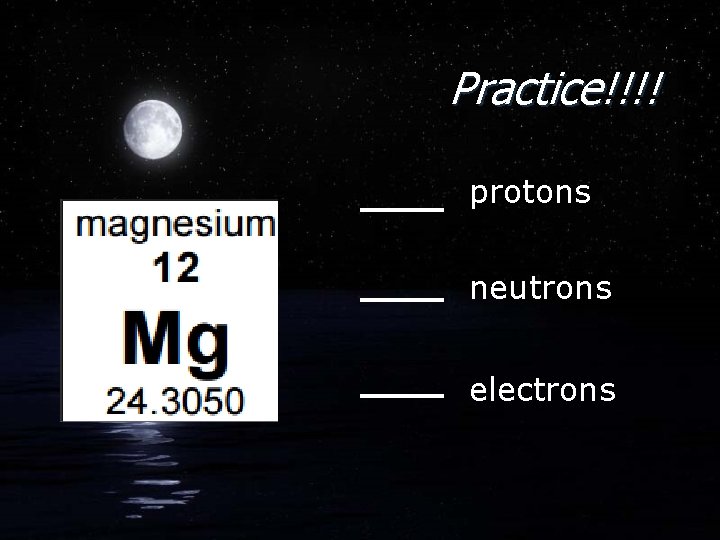
Practice!!!! protons neutrons electrons
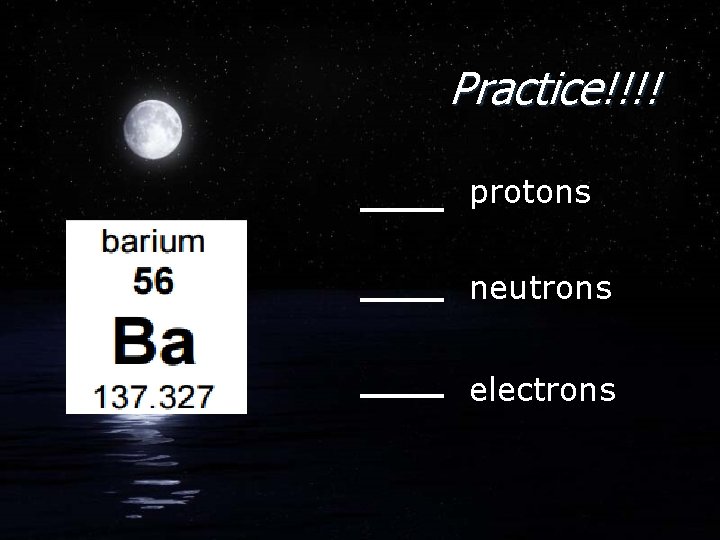
Practice!!!! protons neutrons electrons

Find the number of protons, electrons and neutrons 1) Cl 2) Bh

Practice!!!! 1) Does magnesium have more protons and electrons than titanium? 2) Is barium a heavier element than cesium? 3) Does radium have more electrons than radon? 4) Which has more neutrons, phosphorus or aluminum ?

Ions • Atoms can gain or lose Electrons -- • This ability to gain or lose electrons determines how an element will react to form bonds + • After an element that gains or loses one OR MORE electrons it is called an ION

Positive Ions • Gaining or losing electrons does not affect the number of protons an element has • When an atom loses an electron it becomes POSITIVE (+) • Note: The number of Protons now exceeds the number of Electrons

Negative Ions • When an atom GAINS an electron it becomes NEGATIVE • The number of ELECTRONS now exceeds the number of protons
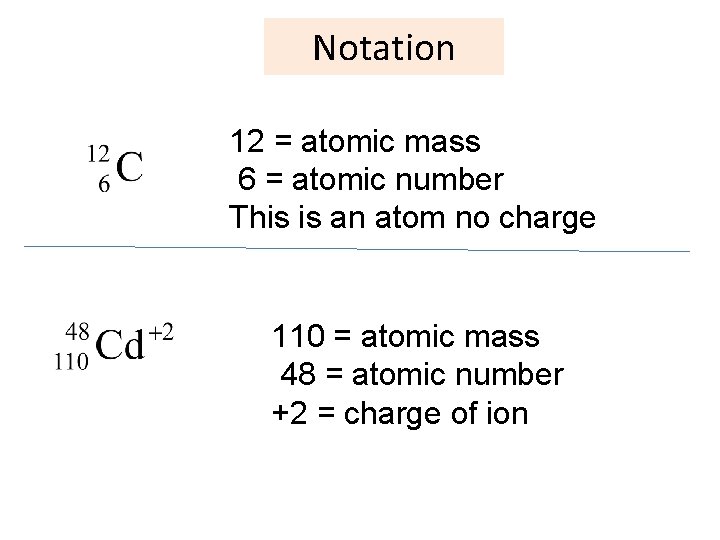
Notation 12 = atomic mass 6 = atomic number This is an atom no charge 110 = atomic mass 48 = atomic number +2 = charge of ion 17

Calculating Subatomic Particles in Ions • How many protons, neutrons, and electrons are in the following ions?
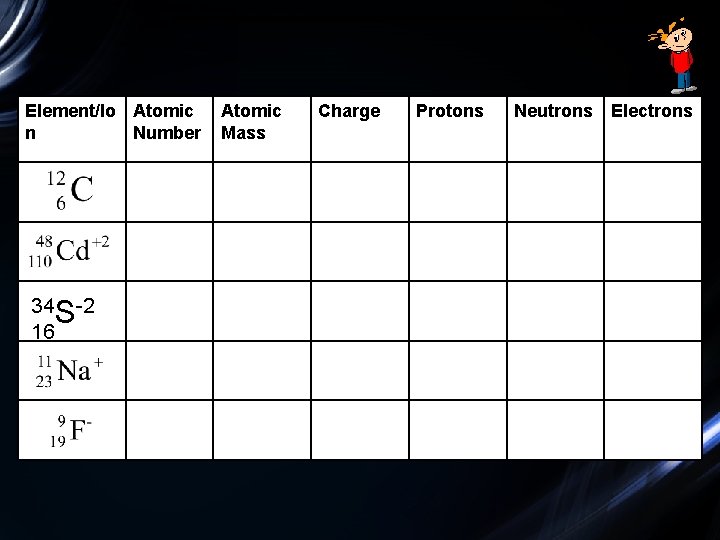
Element/Io Atomic n Number 34 S-2 16 Atomic Mass Charge Protons Neutrons Electrons
PRACTICE
- Slides: 20