Elements and Compounds Pure Substance What does pure
Elements and Compounds

Pure Substance • What does pure mean? – Unmixed – All the same – Uniform • A substance in which there is only one type of particle
Element • A pure substance that cannot be separated into simpler substances by physical or chemical means • MUST be on the periodic table – Gold – Au – Oxygen – O – Helium - He

Element or Not? • Lead? – Yes! #82 • Steel? – No • Water? – No • Copper? – Yes! #29 • Sugar? – No • Brass? – No • Salt? – No • Chlorine? – Yes! #17
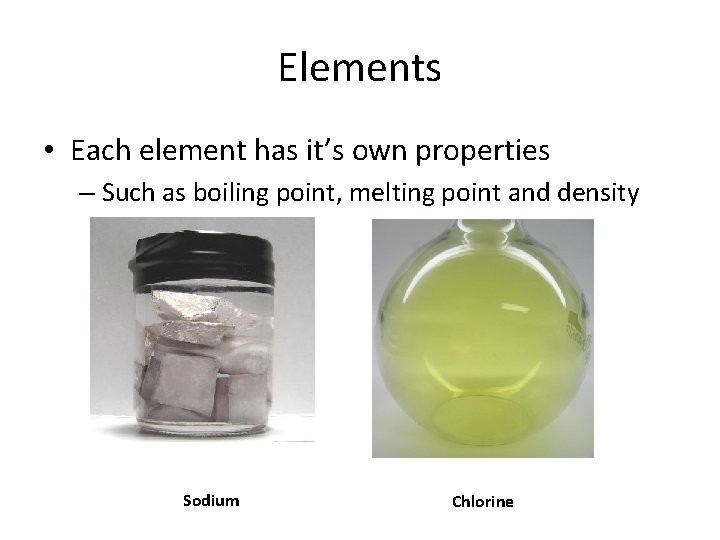
Elements • Each element has it’s own properties – Such as boiling point, melting point and density Sodium Chlorine
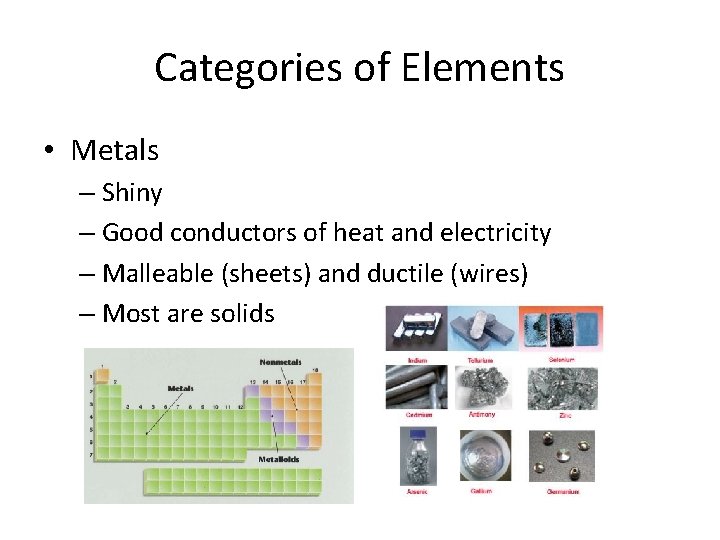
Categories of Elements • Metals – Shiny – Good conductors of heat and electricity – Malleable (sheets) and ductile (wires) – Most are solids
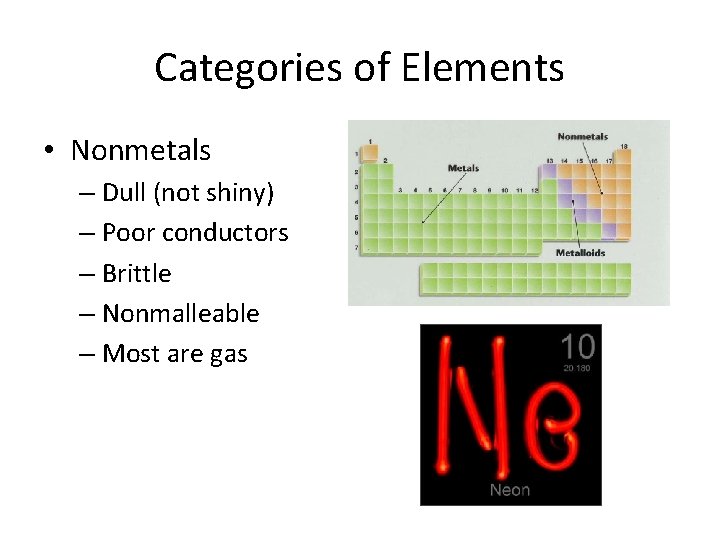
Categories of Elements • Nonmetals – Dull (not shiny) – Poor conductors – Brittle – Nonmalleable – Most are gas
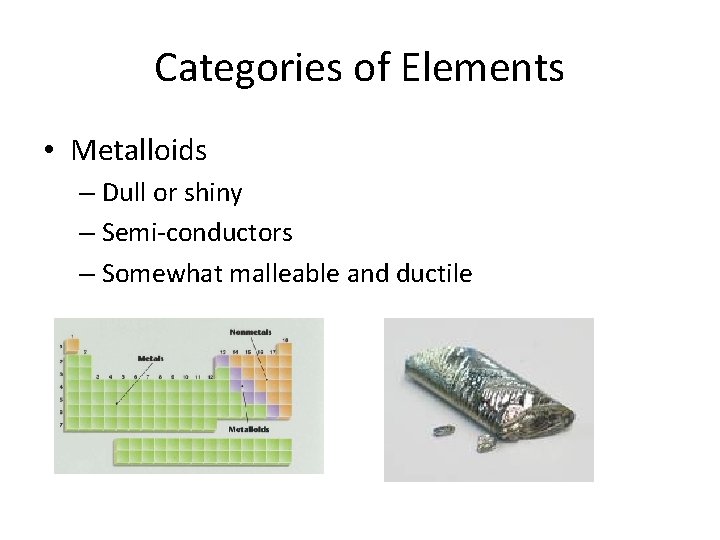
Categories of Elements • Metalloids – Dull or shiny – Semi-conductors – Somewhat malleable and ductile
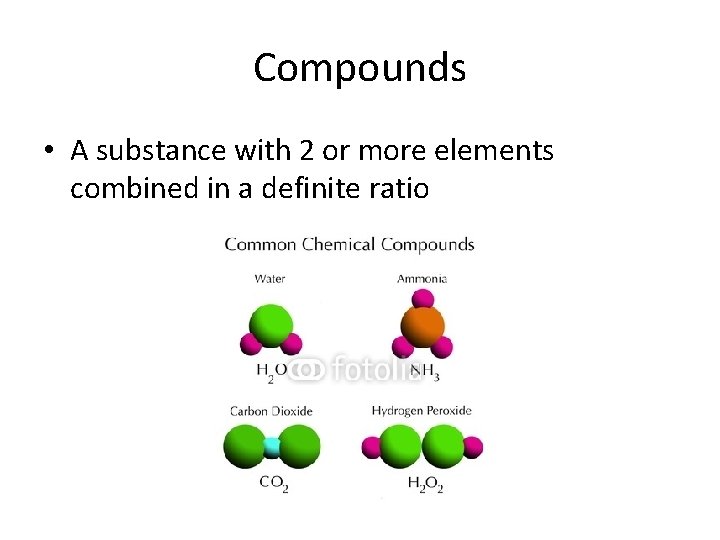
Compounds • A substance with 2 or more elements combined in a definite ratio
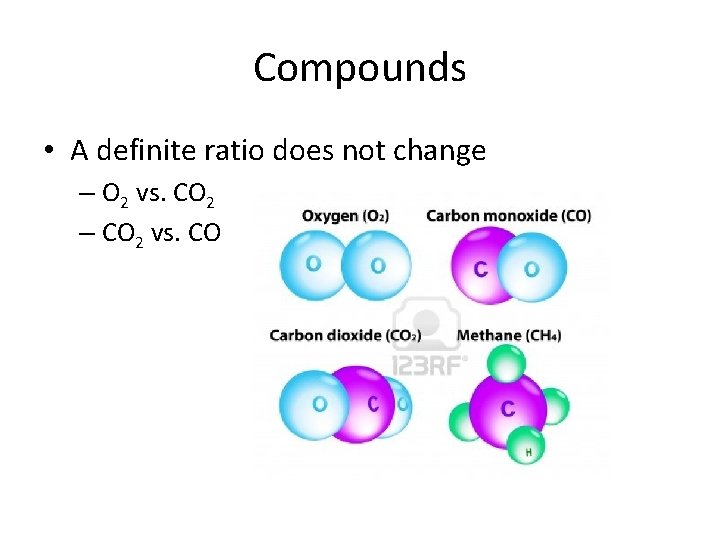
Compounds • A definite ratio does not change – O 2 vs. CO 2 – CO 2 vs. CO

Compounds • Compounds have a unique set of properties • Different than the elements that make it up = + Sodium Chlorine Sodium Chloride (Salt)
- Slides: 11