Elements and Compounds Atoms Most basic unit of

Elements and Compounds
Atoms § Most basic unit of matter to contain a chemical property. § The chemical properties of an atom are determined by the number of protons found in the nucleus.

Elements § Elements are pure substances compose of only 1 type of atom. § Ex. Gold is an element. Even though there are many atoms in this chunk of gold, it is only made of gold atoms.

Compounds When 2 or more atoms of different elements bond together they form a compound. This compound now has different chemical properties than the atoms that make up the compound.

Example § § § Sodium (Na) is a highly reactive metal. Chlorine (Cl) is a toxic gas. But when they combine, they form the easily dissolvable and non-toxic compound called Sodium Chloride (Na. Cl) or commonly called table salt.
Chemical Formulas § A compound is represented by a chemical formula. § The symbols and the amounts of each atoms are represented in the chemical formula. § For the previous example…

§ We combined: § 1 atom of Sodium (Na) §+ § 1 atom of Chlorine (Cl) § Na + Cl Na. Cl § This formula says that the compound is made of 1 atom of sodium and 1 atom of chlorine.

But what if you have more than one type of atom in a compound? ? § Ammonia is a common cleaning liquid. § It is composed of: § 1 Nitrogen Atom §+ § 3 Hydrogen Atoms § N+ H +H+ H NH 3 § For every atom of N there is 3 atoms of hydrogen
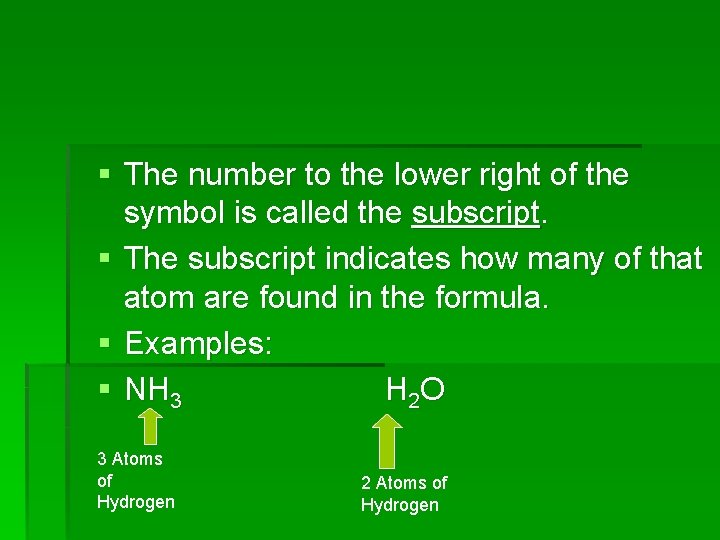
§ The number to the lower right of the symbol is called the subscript. § The subscript indicates how many of that atom are found in the formula. § Examples: § NH 3 H 2 O 3 Atoms of Hydrogen 2 Atoms of Hydrogen

You Try This One!! § Sugar is a common food. § Sugar contains: § 6 atoms of Carbon (C) § 12 atoms of Hydrogen (H) § 6 atoms of Oxygen (O) § Write the chemical formula for sugar!!

§ Chemical Formula for Sugar § C 6 H 12 O 6

Complex Formulas § Sometimes chemical formulas contain many atoms § CH 3 COOH (Vinegar) § C 12 H 17 N 4 OS (Vitamin B 1) Some symbols may be separated from other symbols by parentheses: Mg (OH)2 (Milk of Magnesia) They are written this way to indicate there are two OH groups bonded to one Mg.

Mg OH OH In order to calculate the total number of atoms in the compound you must multiply the atoms inside the ( ) by the number outside the ( ).

§ Mg (OH)2 In this chemical formula there is: 1 Mg Atom 2 Oxygen Atoms 2 Hydrogen Atom

You Try! § How many of each atoms are in Na (Cl. O 2)2
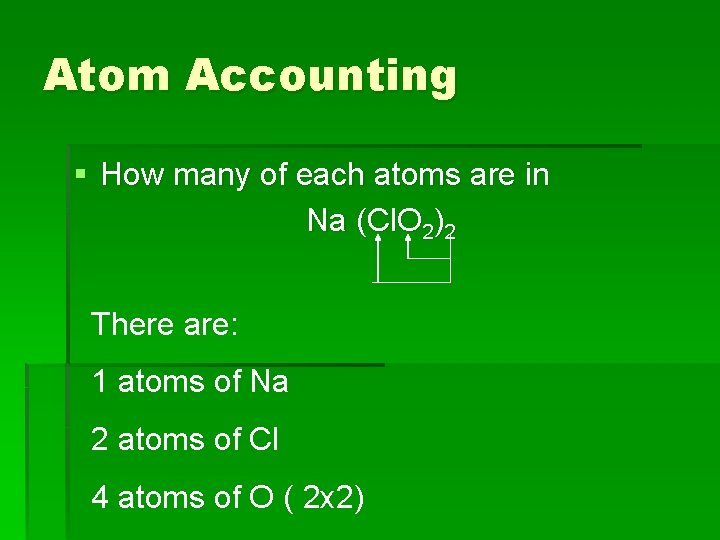
Atom Accounting § How many of each atoms are in Na (Cl. O 2)2 There are: 1 atoms of Na 2 atoms of Cl 4 atoms of O ( 2 x 2)

Coefficients § What if we had two molecules of Na (Cl. O 2)2 ? § How many atoms would you have of each?

§ When you have more than 1 molecule of a compound you write it with a coefficient. § EX. 2 Na (Cl. O 2)2 Coefficient

Counting Atoms § To get the total number of atoms, you multiply ALL the atoms by the coefficient. § For example: § 2 H 2 O § You have 2 atoms of hydrogen and 1 atom of oxygen for each molecule. § So: 2 X 2 H’s 2 X 1 O’s Total: 4 H’s and 2 O’s
- Slides: 19