Electrostatics Electrostatics Sections Covered Honors Physics Chapters 20












- Slides: 25

Electrostatics

Electrostatics Sections Covered – Honors Physics – Chapters 20 and 21 Topics Covered – Electrical Charges – Calculating Charge – Transfer of Charge/Conservation of Charge – Insulators and Conductors – Coulomb’s Law – Lightning

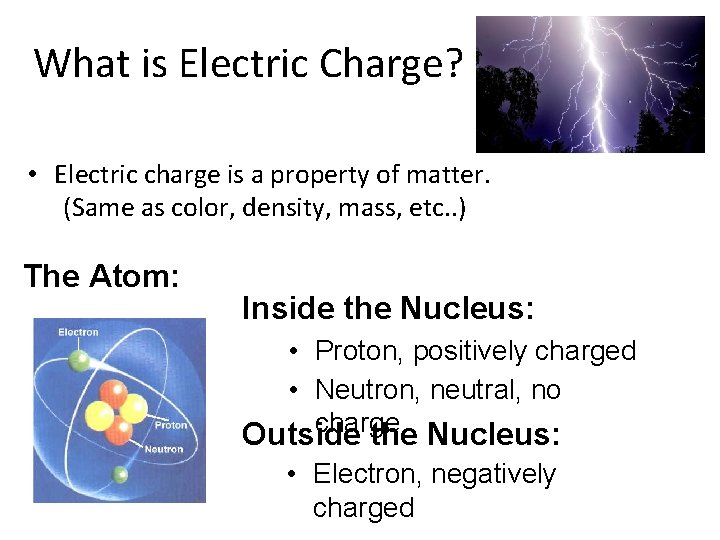
What is Electric Charge? • Electric charge is a property of matter. (Same as color, density, mass, etc. . ) The Atom: Inside the Nucleus: • Proton, positively charged • Neutron, neutral, no charge Outside the Nucleus: • Electron, negatively charged

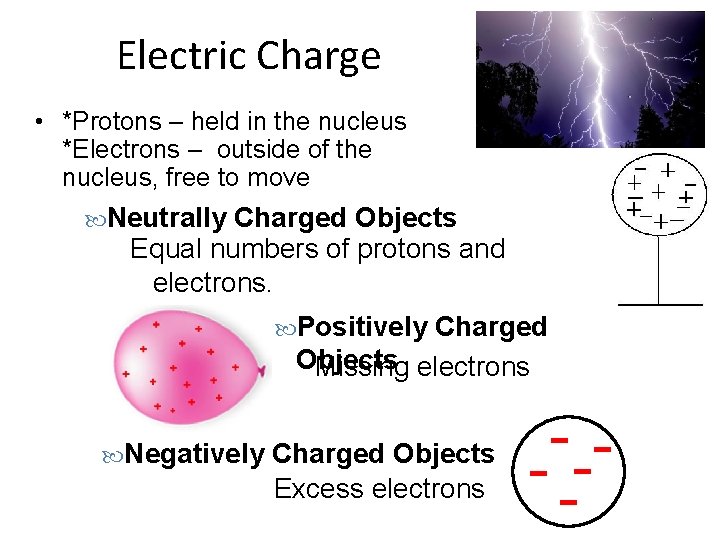
Electric Charge • *Protons – held in the nucleus *Electrons – outside of the nucleus, free to move Neutrally Charged Objects Equal numbers of protons and electrons. Positively Charged Objects Missing electrons Negatively Charged Objects Excess electrons

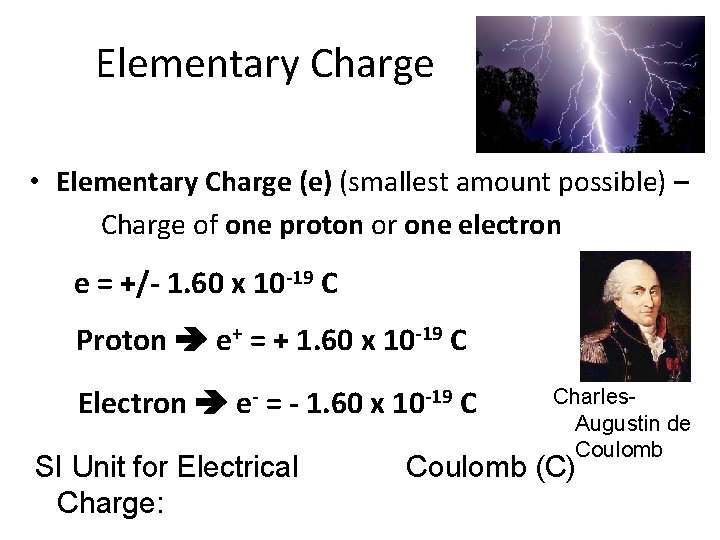
Elementary Charge • Elementary Charge (e) (smallest amount possible) – Charge of one proton or one electron e = +/- 1. 60 x 10 -19 C Proton e+ = + 1. 60 x 10 -19 C Electron e- = - 1. 60 x 10 -19 C SI Unit for Electrical Charge: Charles. Augustin de Coulomb (C)

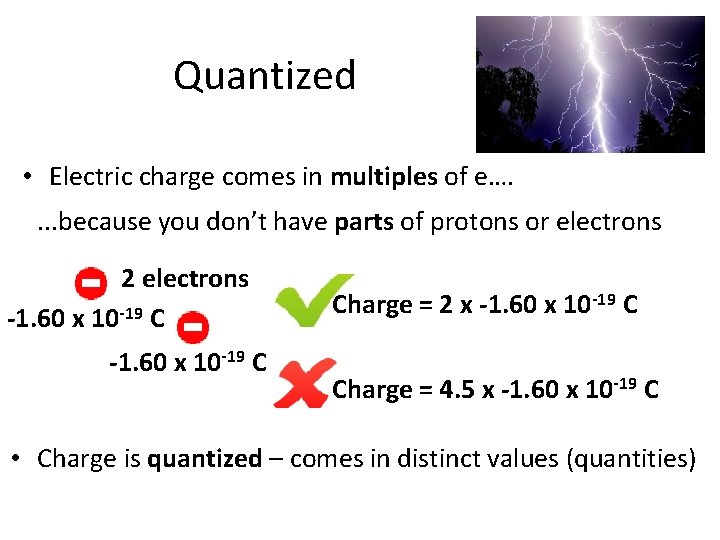
Quantized • Electric charge comes in multiples of e…. . because you don’t have parts of protons or electrons 2 electrons -1. 60 x 10 -19 C Charge = 2 x -1. 60 x 10 -19 C Charge = 4. 5 x -1. 60 x 10 -19 C • Charge is quantized – comes in distinct values (quantities)

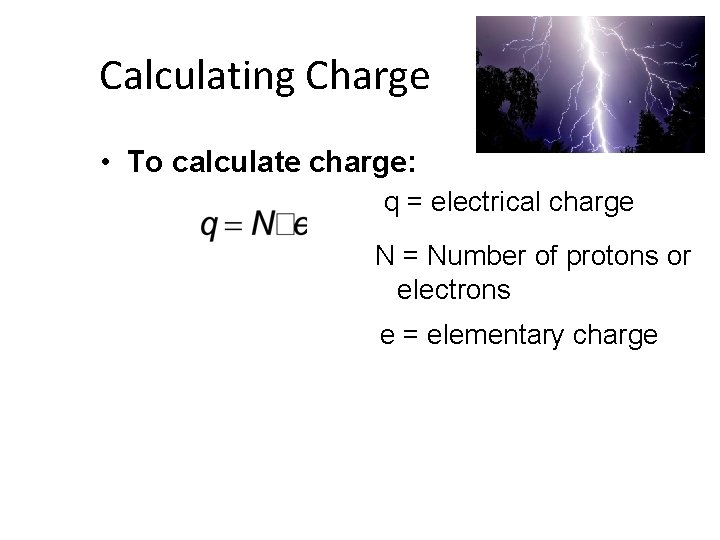
Calculating Charge • To calculate charge: q = electrical charge N = Number of protons or electrons e = elementary charge

Example 1 An object contains 5. 50 x 1014 protons and 5. 00 x 1014 electrons. What is the overall charge on this object?

Example 2 A sphere has a charge of 4. 00 C. How many electrons must be added so the charge becomes -2. 00 C?

Example 3 A metal sphere initially has a charge of -15. 0 C. What will be the net charge on the sphere if 4. 65 x 1014 electrons are also added?

Conductors and Insulators • Conductors - Substances that allow electrons to transfer easily. Examples: Copper, iron, steel, brass, gold…metals Insulators - Substances that do not allow electrons to transfer easily. Examples: Wood, plastic, glass, oil, ceramics

Is Water Conductive? • Thoughts? SURPRISE! Pure water is actually not conductive But, the minerals and other salts found in water make it a conductive substance.

How Objects Gain/Lose Charge • Three methods: 1. Charging by Contact 2. Polarization 3. Induction

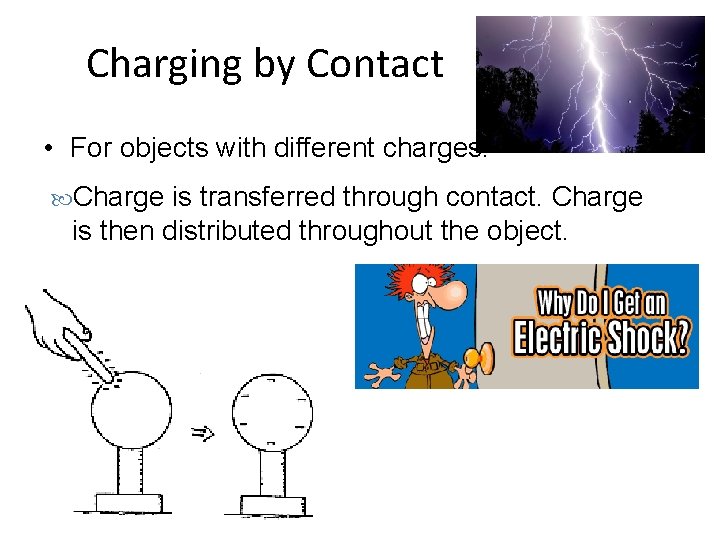
Charging by Contact • For objects with different charges. Charge is transferred through contact. Charge is then distributed throughout the object.

Charging by Contact – Real Life Lightning Static shock

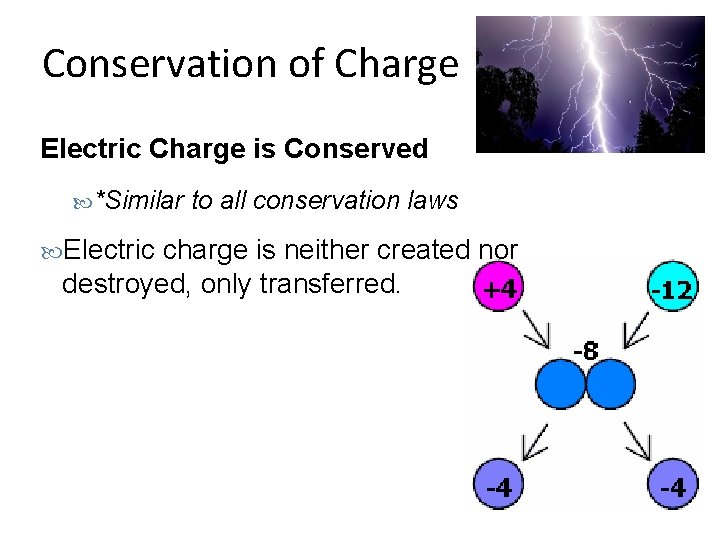
Conservation of Charge Electric Charge is Conserved *Similar Electric to all conservation laws charge is neither created nor destroyed, only transferred.

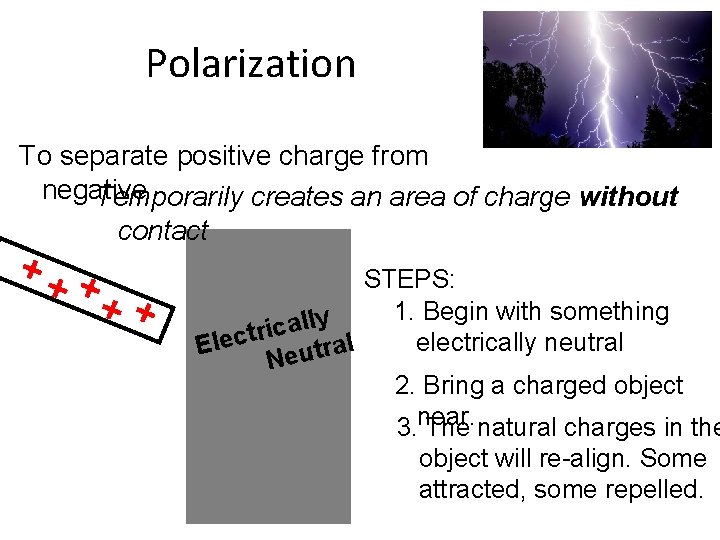
Polarization To separate positive charge from negative Temporarily creates an area of charge without contact STEPS: 1. Begin with something y l l a ric t c e l electrically neutral l E a r t u Ne 2. Bring a charged object 3. near. The natural charges in the object will re-align. Some attracted, some repelled.

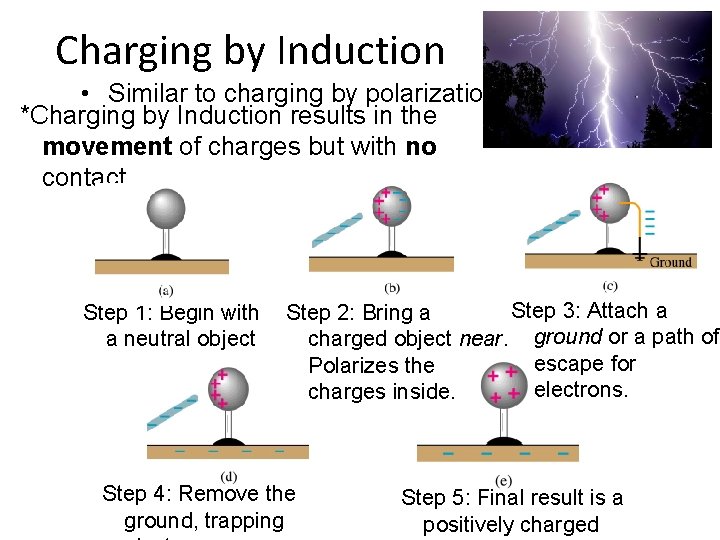
Charging by Induction • Similar to charging by polarization *Charging by Induction results in the movement of charges but with no contact. Step 1: Begin with a neutral object Step 3: Attach a Step 2: Bring a charged object near. ground or a path of escape for Polarizes the electrons. charges inside. Step 4: Remove the ground, trapping Step 5: Final result is a positively charged

Electrostatic Induction – Real Life Uses…. Precautions Against…

Example 4 – Review Problem *There’s a reason for this, I promise Find the Fx and Fy for the forces shown.

Laws of Attraction …and Repulsion *Opposites attract (protons and electrons) *Likes repel (proton and proton, electron and electron) The closer the charges are, the stronger the force of attraction or repulsion *But how much attraction and/or repulsion is there?

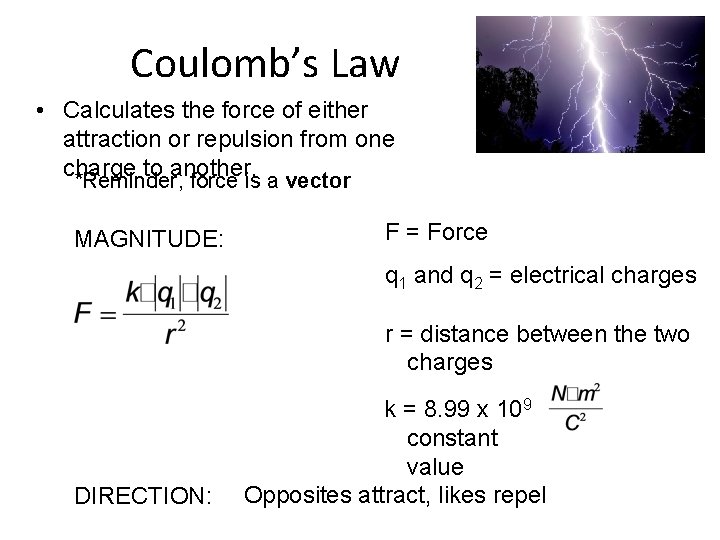
Coulomb’s Law • Calculates the force of either attraction or repulsion from one charge to another. *Reminder, force is a vector MAGNITUDE: F = Force q 1 and q 2 = electrical charges r = distance between the two charges DIRECTION: k = 8. 99 x 109 constant value Opposites attract, likes repel

Example 5 The nucleus of a helium atom contains two protons that are separated by a distance of 3. 00 x 10 -15 m. A. How much force do they exert on each other? B. If each proton has a mass of 1. 67 x 10 -27 kg, and utilizing the force found in part A, what would be the acceleration of either of the protons?

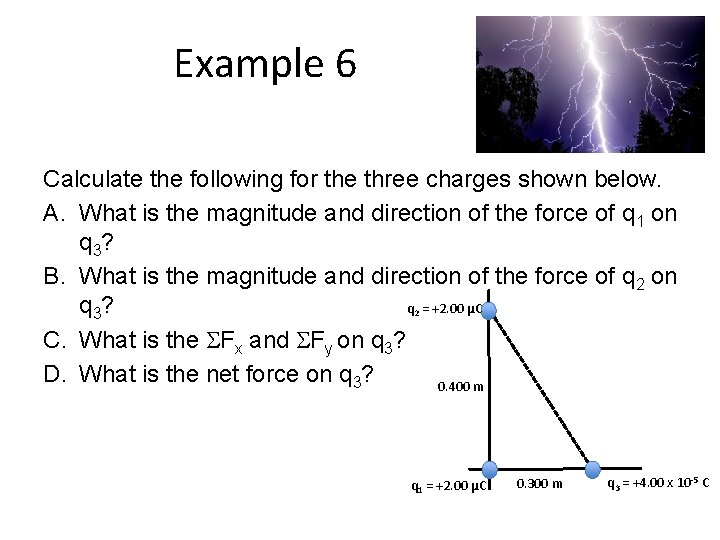
Example 6 Calculate the following for the three charges shown below. A. What is the magnitude and direction of the force of q 1 on q 3? B. What is the magnitude and direction of the force of q 2 on q = +2. 00 μC q 3? C. What is the Fx and Fy on q 3? D. What is the net force on q 3? 0. 400 m 2 q 1 = +2. 00 μC 0. 300 m q 3 = +4. 00 x 10 -5 C

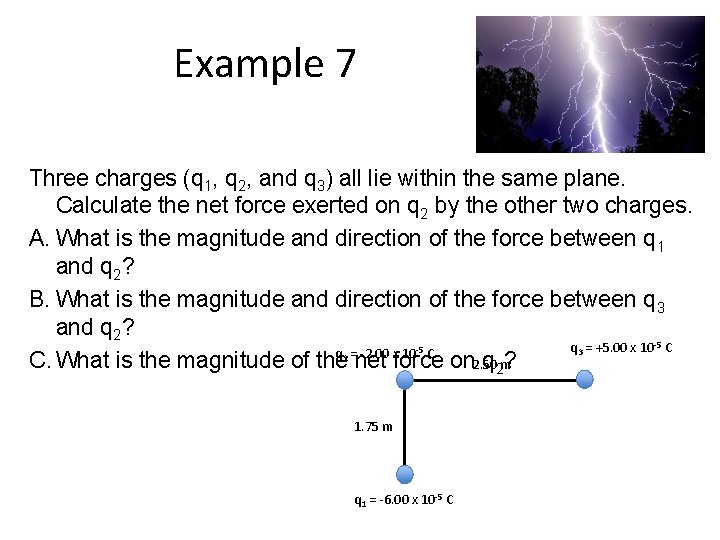
Example 7 Three charges (q 1, q 2, and q 3) all lie within the same plane. Calculate the net force exerted on q 2 by the other two charges. A. What is the magnitude and direction of the force between q 1 and q 2? B. What is the magnitude and direction of the force between q 3 and q 2? q 3 = +5. 00 x 10 -5 C q 2 = -2. 00 x 10 -5 C C. What is the magnitude of the net force on 2. 50 q 2 m? 1. 75 m q 1 = -6. 00 x 10 -5 C