Electrostatics Electricity in one form or another is
- Slides: 6

Electrostatics

Electricity in one form or another is all around you: Lightning from the sky, spark from you hand on a dry day, etc. Electrostatics is electricity at rest. It involves: -Electric Charges -Forces between them -Their behavior in materials • Electric Forces and Charges Unlike the force of gravity that only attracts, electrical forces not only attract but also repel. Electrical forces arise from particles in atoms -Every atom has a positively charged nucleus surrounded by negatively charged electrons. -All electrons are identical, same mass and same identical negatively charge as other electrons - Nucleus: Composed of protons and neutrons. Protons have 2000 times the mass of electrons and are positively charged. Neutrons have no charge. - As many protons as electrons, so the atom has zero charge. Like charges repel, opposite charges attract.

• Conservation of Charge Electrons and protons have electric charge. In a neutral atom: number of electrons = number of protons # e- > # p+ Negative ion # e- < # p+ Positive ion An object that has unequal numbers of electrons and protons is electrically charged. If it has more electrons than protons, the object is negatively charged. If it has fewer electrons than protons, then it is positively charged. • Coulomb’s Law Newton’s law of gravitation: F = G m 1 m 2 d 2 (Force of gravitation) The electrical force between any two objects obeys to a similar inverse-square relationship with distance. F = k q 1 q 2 q: electric charge [coulomb] [C] d 2 k = 9 x 109 N m 2/C 2

• Coulomb’s Law (cont’d) Comparing Newton’s and Coulomb’s Laws. The greatest difference between gravitational and electrical forces is that while gravity only attracts, electrical forces may either attract or repel. • Conductors and Insulators Electrons are more easily moved in some materials than in others. Outer electrons in the atoms in a metal are not anchored to the nuclei of particular atoms and they are free to roam in the material. Metals are good conductors. Electrons in rubber and glass for example are tightly bound and remain with particular atoms. They are not free to wander about other atoms in the material. Such materials are good insulators

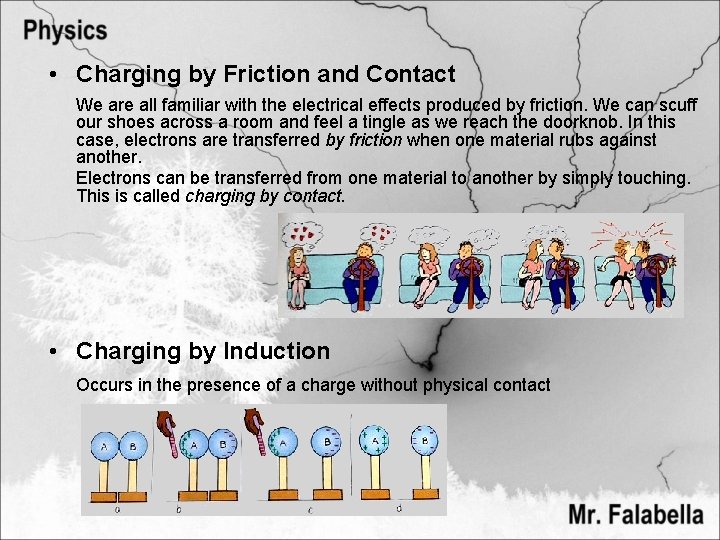
• Charging by Friction and Contact We are all familiar with the electrical effects produced by friction. We can scuff our shoes across a room and feel a tingle as we reach the doorknob. In this case, electrons are transferred by friction when one material rubs against another. Electrons can be transferred from one material to another by simply touching. This is called charging by contact. • Charging by Induction Occurs in the presence of a charge without physical contact

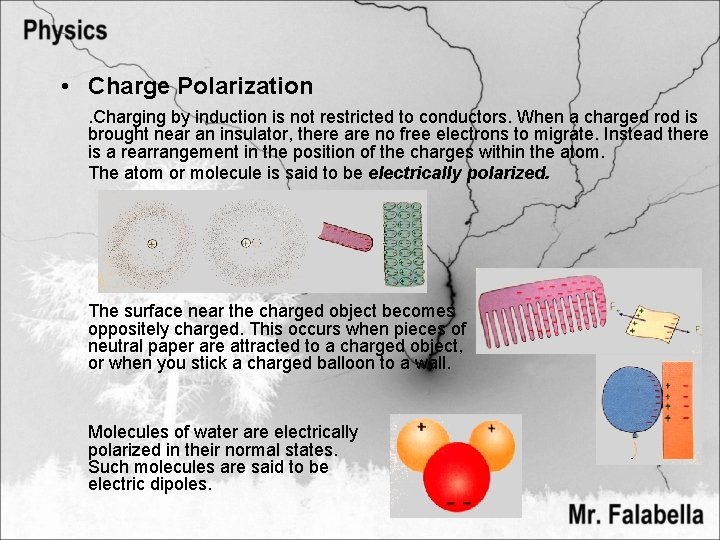
• Charge Polarization. Charging by induction is not restricted to conductors. When a charged rod is brought near an insulator, there are no free electrons to migrate. Instead there is a rearrangement in the position of the charges within the atom. The atom or molecule is said to be electrically polarized. The surface near the charged object becomes oppositely charged. This occurs when pieces of neutral paper are attracted to a charged object, or when you stick a charged balloon to a wall. Molecules of water are electrically polarized in their normal states. Such molecules are said to be electric dipoles.