Electrostatics Electricity Electricity is all around us Cell








- Slides: 13

Electrostatics

Electricity • Electricity is all around us: – Cell phones – Computers – Living organisms – Lightening • Electrostatics – “electricity at rest, static electricity” (Hewitt 407). – “involves electric charges, the forces between them, the aura that surrounds them, and their behavior in materials” (Hewitt 407)

Electrical Forces • What forces govern nature? – We have definitely heard of gravitational force. Is there anything stronger? • Yes! It is called the “Electric Force. ” • Electrical force – “The force that one charge exerts on another. When the charges are the same sign, they repel; when the charges are opposite, they attract. ” (Hewitt G-5) – Like repels like, opposites attract

Electrical Forces • There are positive and negative charges – Most matter though, is electrically neutral (no charge) • The positive charges balance out the negative charges

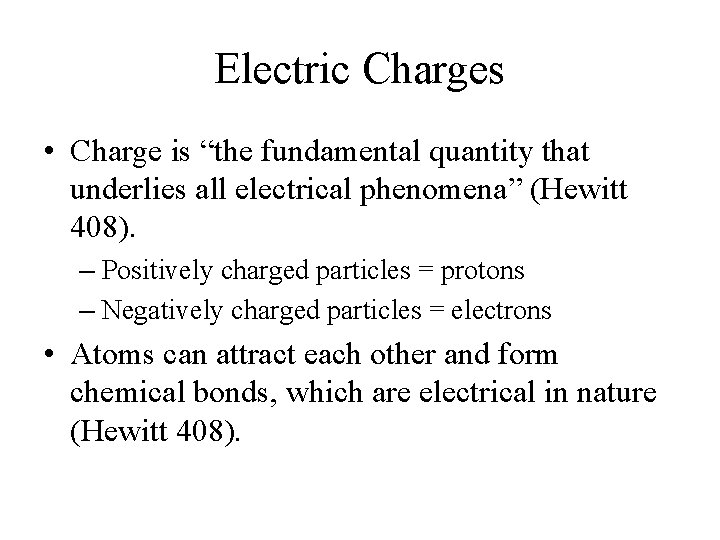
Electric Charges • Charge is “the fundamental quantity that underlies all electrical phenomena” (Hewitt 408). – Positively charged particles = protons – Negatively charged particles = electrons • Atoms can attract each other and form chemical bonds, which are electrical in nature (Hewitt 408).

Electric Charges • Remember the structure of the atom: – Protons (positive charge) and neutrons (no charge) are in the nucleus • Nucleus is positively charged • Protons and neutrons are much more massive than electrons (with neutrons being slightly more massive) – Electrons (negatively charge – charge equal in magnitude but opposite in sign of that of a proton) orbit about the nucleus • All electrons are the same in terms of mass and “quantity of negative charge” (Hewitt 409) – The number of protons typically equals the number of electrons in an atom (no net charge of the atom) (Hewitt 409)

Electric Charges • Even though the electrons surround the positively charge nucleus, the electrons do not spiral into the nucleus – Quantum mechanics can help explain this • Electrons behave as waves with a fixed wavelength (Hewitt 409)

Charge is Conserved! • Conservation of Charge – “The principle that net electric charge is neither created nor destroyed but is transferrable from one object to another” (Hewitt G-3) • “whenever something is charged, no electrons are created or destroyed” (Hewitt 409)

Charge is Conserved! • When an atom loses or gains an electron, it becomes an ion – Positive ion (cation) – more protons than electrons – Negative ion (anion) – more electrons than protons • Any time that an object is charged, it “has an excess or deficiency of some whole number of electrons” (Hewitt 410) – Charge is quantized!

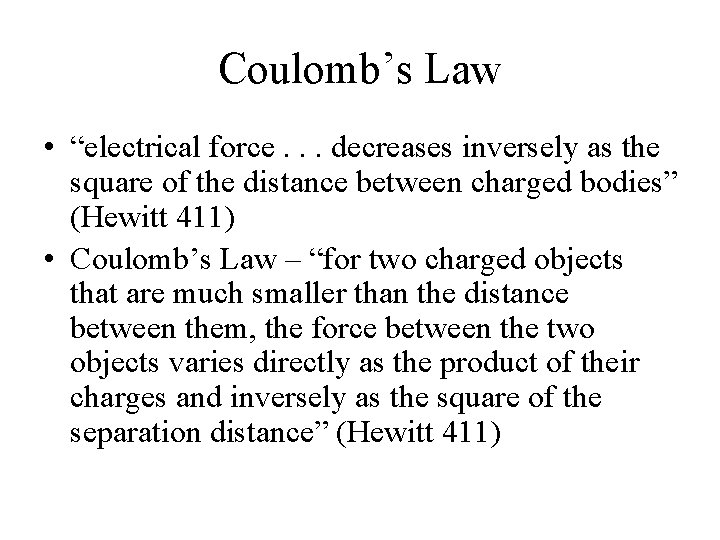
Coulomb’s Law • “electrical force. . . decreases inversely as the square of the distance between charged bodies” (Hewitt 411) • Coulomb’s Law – “for two charged objects that are much smaller than the distance between them, the force between the two objects varies directly as the product of their charges and inversely as the square of the separation distance” (Hewitt 411)

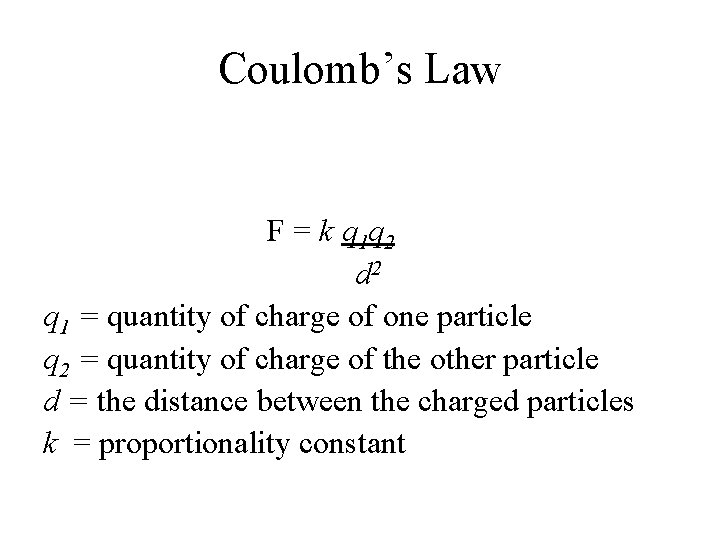
Coulomb’s Law F = k q 1 q 2 d 2 q 1 = quantity of charge of one particle q 2 = quantity of charge of the other particle d = the distance between the charged particles k = proportionality constant

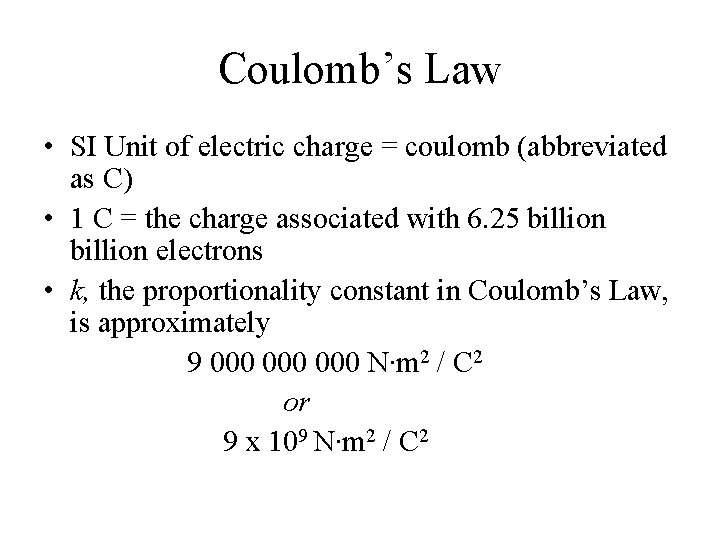
Coulomb’s Law • SI Unit of electric charge = coulomb (abbreviated as C) • 1 C = the charge associated with 6. 25 billion electrons • k, the proportionality constant in Coulomb’s Law, is approximately 9 000 000 N∙m 2 / C 2 or 9 x 109 N∙m 2 / C 2

Works Cited Hewitt, Paul G. Conceptual Physics. Twelfth edition, Pearson, 2015, 2010, 2006.