Electrostatics Chapter 32 Electrical Forces and Charges l








- Slides: 15

Electrostatics Chapter 32

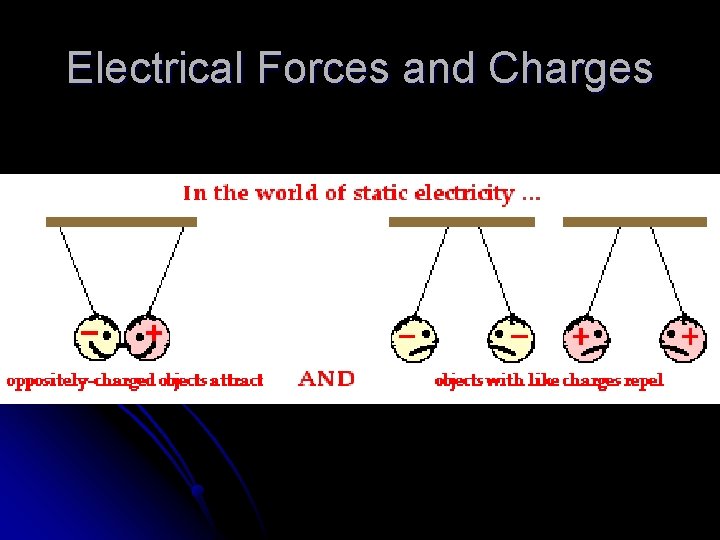
Electrical Forces and Charges l l 1. 2. 3. 4. Electrical Force – a force that one charge exerts on another Charge – the fundamental electric property to which the mutual attractions or repulsions between electrons or protons is attributed Atom Facts Every atom has a positively charged nucleus All electrons are identical; each has the same mass and the same quantity of negative charge as every other electron The nucleus is composed of protons and neutrons; all protons are identical and all neutrons are identical Atoms usually have as many electrons as protons, so the atom has zero net charge Like charges repel; opposite charges attract

Electrical Forces and Charges

Conservation of Charge l l l l In a neutral atom, there as many electrons as protons, so there is no net charge If an electron is removed from the atom, the atom is no longer neutral and is said to be positively charged A charged atom is called an ion A positive ion has a net positive charge, it has lost one or more electrons A negative ion has a net negative charge, it has gained one or more extra electrons The outermost electrons of many atoms are bound very loosely and can be easily dislodged from the atom Conservation of Charge – the principle that net electronic charge is neither created nor destroyed but is transferable from one material to another

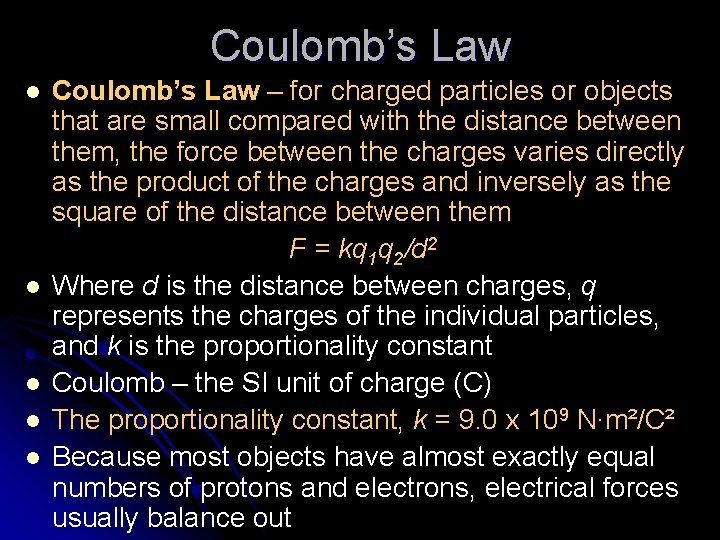
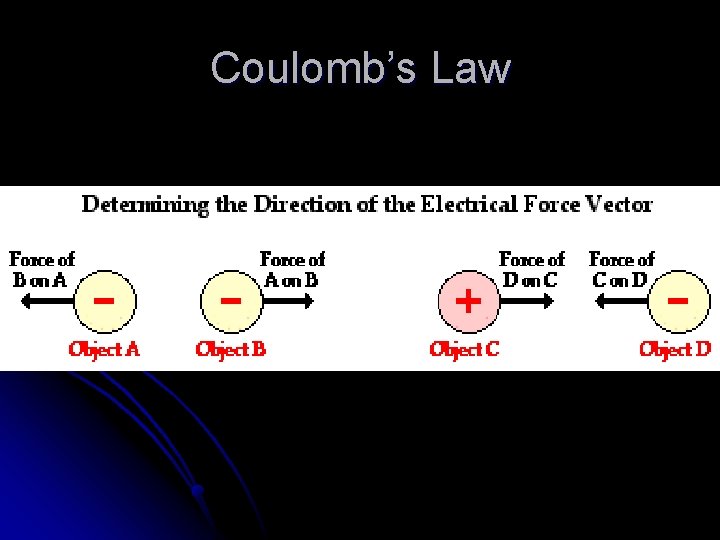
Coulomb’s Law l l l Coulomb’s Law – for charged particles or objects that are small compared with the distance between them, the force between the charges varies directly as the product of the charges and inversely as the square of the distance between them F = kq 1 q 2/d 2 Where d is the distance between charges, q represents the charges of the individual particles, and k is the proportionality constant Coulomb – the SI unit of charge (C) The proportionality constant, k = 9. 0 x 109 N∙m²/C² Because most objects have almost exactly equal numbers of protons and electrons, electrical forces usually balance out

Coulomb’s Law

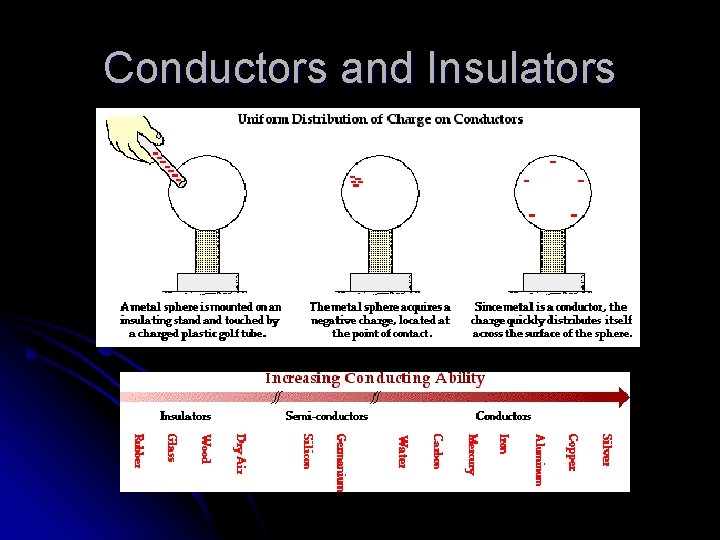
l l l Conductors and Insulators Conductors – material, usually a metal, through which electric charge can flow Insulators – a material that is a poor conductor of electricity All substances can be arranged in order of their ability to conduct electric charges (top = conductors, bottom = insulators) Whether a substance is classified as a conductor or an insulator depends on how tightly the atoms of the substance hold their electrons Semiconductors – material that can be made to behave as either a conductor or an insulator of electricity Superconductors – material that has infinite conductivity at very low temperatures, so that charge flows through it without resistance

Conductors and Insulators

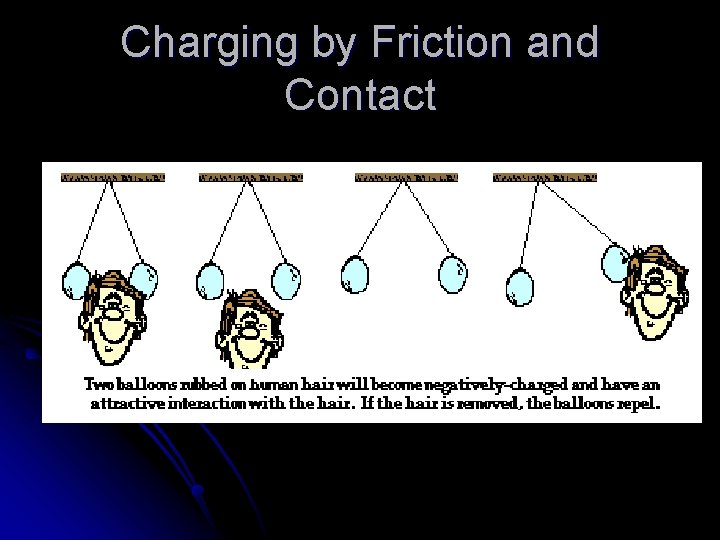
Charging by Friction and Contact Electrons can be transferred by friction, when one material rubs against another l Electrons can be transferred from one material to another by simply touching l If a charged rod is placed in contact with a neutral object, some charge will transfer to the neutral object l If the object is a good conductor, the charge will spread to all parts of its surface because like charges repel l

Charging by Friction and Contact

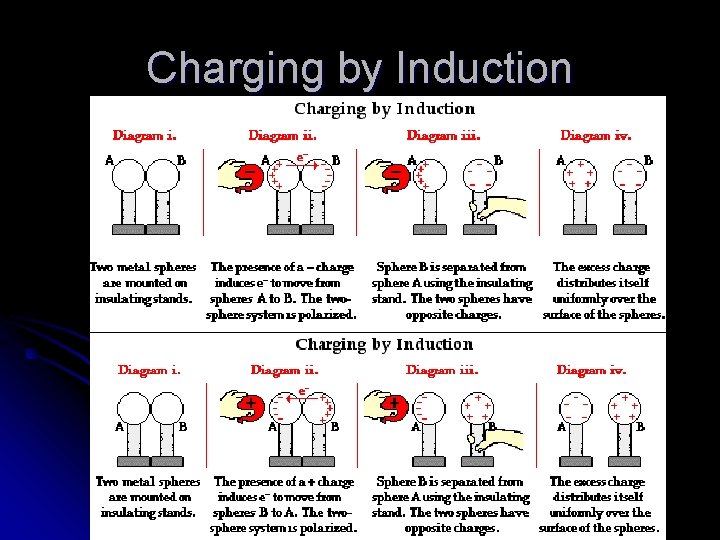
Charging by Induction l l l If we bring a charged object near a conducting surface, even without physical contact, electrons will move in the conducting surface Induced – term that is applied to electric charge that has been redistributed on an object because of the presence of a charged object nearby Induction – the charging of an object without direct contact Grounding – allowing charges to move freely along a connection between a conductor and the ground Charging by induction occurs during thunder storms. The negatively charged bottoms of clouds induce a positive charge on the surface of Earth below. Most lightning is an electrical discharge between oppositely charged parts of clouds

Charging by Induction

Charge Polarization When a charged rod is brought near an insulator, there are no free electrons to migrate throughout the material l There is a rearrangement of the positions of charges within the atoms and molecules themselves l Electrically Polarized – term applied to an atom or molecule in which the charges are aligned so that one side is slightly more positive or negative than the opposite side l

Charge Polarization

Assignment Read Chapter 32 (pg. 500 -514) l Do #26 -36 (pg. 516) l Appendix F #1 -10 (pg. 686 -687) l