Electrostatics 41415 PREFIXES Factor Prefix Symbol 1012 tera

Electrostatics 4/14/15
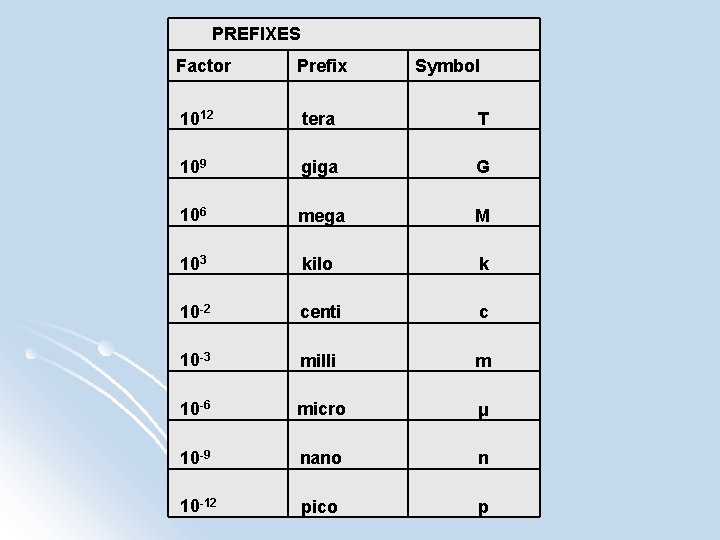
PREFIXES Factor Prefix Symbol 1012 tera T 109 giga G 106 mega M 103 kilo k 10 -2 centi c 10 -3 milli m 10 -6 micro μ 10 -9 nano n 10 -12 pico p

Coulomb's Law

Example l If there is an electrical force of 0. 0345 N between two charges of -632 n. C what is there separation distance?

Homework l Electrostatic WS due Wed. 4/15/15
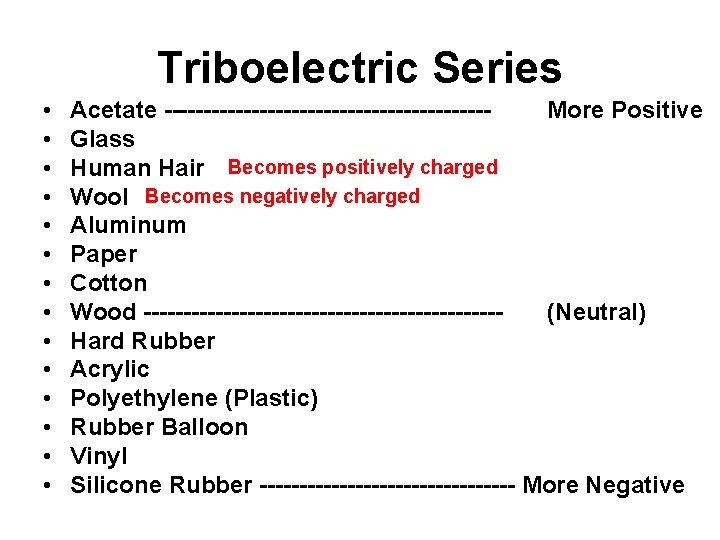
Triboelectric Series • • • • Acetate --------------------More Positive Glass Human Hair Becomes positively charged Wool Becomes negatively charged Aluminum Paper Cotton Wood ----------------------(Neutral) Hard Rubber Acrylic Polyethylene (Plastic) Rubber Balloon Vinyl Silicone Rubber ---------------- More Negative

What Happened in the Lab?
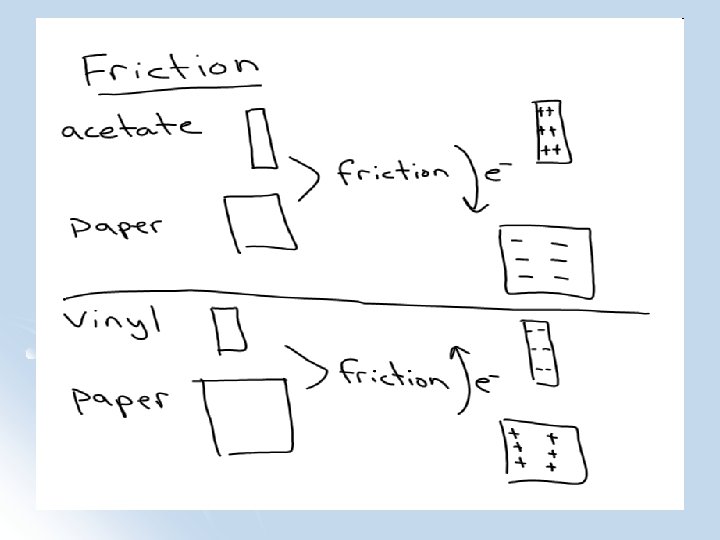
Types of particles Electron: a negatively charged “particle” in the atom’s shell or outer layers. (mass is about 1/2000 of a proton and neutron) l Proton: a positively charged “particle” in the atom’s nucleus. l Neutron: a “particle” with no charge in the atom’s nucleus. l Electrons have a small mass compared to protons and neutrons (which have about the same mass) l
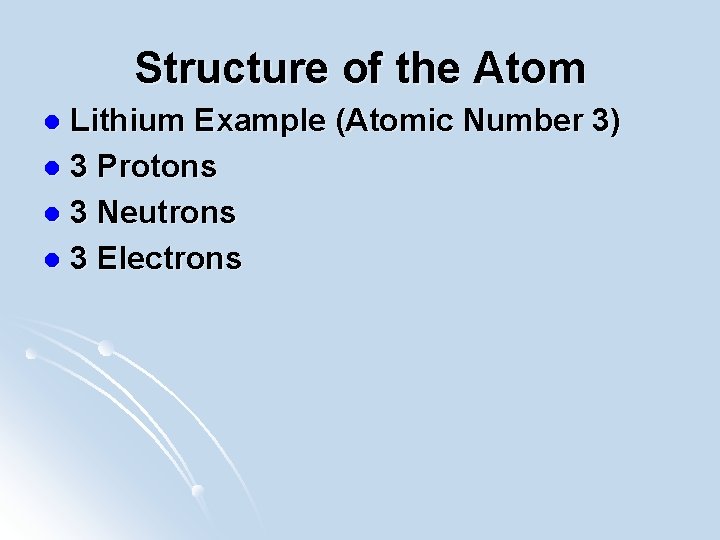
Structure of the Atom Lithium Example (Atomic Number 3) l 3 Protons l 3 Neutrons l 3 Electrons l
Electric Charges and Force l Electrons are the particles that transfer (do the moving) in atoms and molecules l Electric charges are conserved l Electric force l. Like charges repel l. Opposite charges attract
Ions Due to the net transfer (movement) of electrons l If the atom has more electrons than protons it is a negative ion l If the atom has more protons than electrons it is a positive ion l
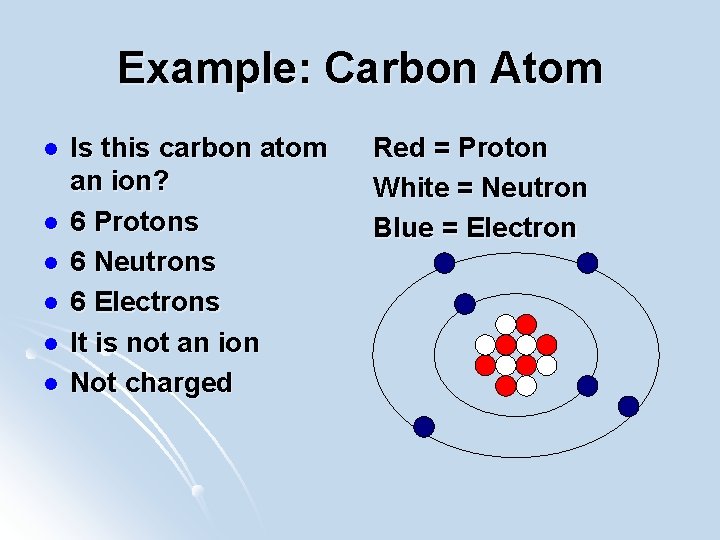
Example: Carbon Atom l l l Is this carbon atom an ion? 6 Protons 6 Neutrons 6 Electrons It is not an ion Not charged Red = Proton White = Neutron Blue = Electron
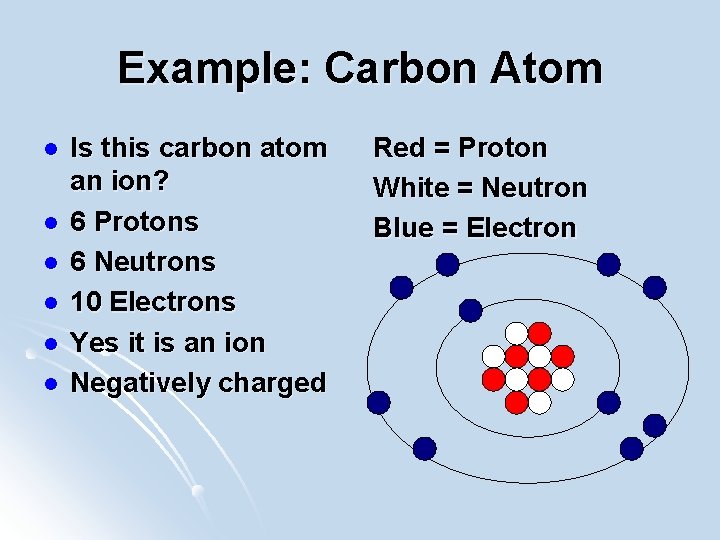
Example: Carbon Atom l l l Is this carbon atom an ion? 6 Protons 6 Neutrons 10 Electrons Yes it is an ion Negatively charged Red = Proton White = Neutron Blue = Electron
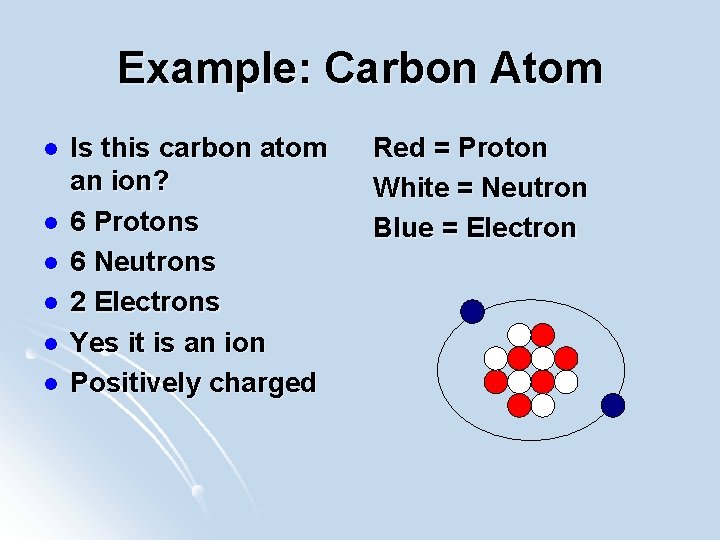
Example: Carbon Atom l l l Is this carbon atom an ion? 6 Protons 6 Neutrons 2 Electrons Yes it is an ion Positively charged Red = Proton White = Neutron Blue = Electron

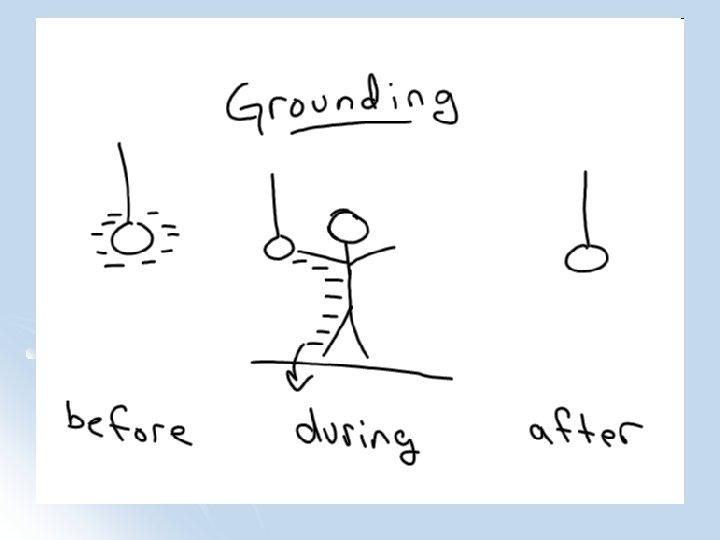
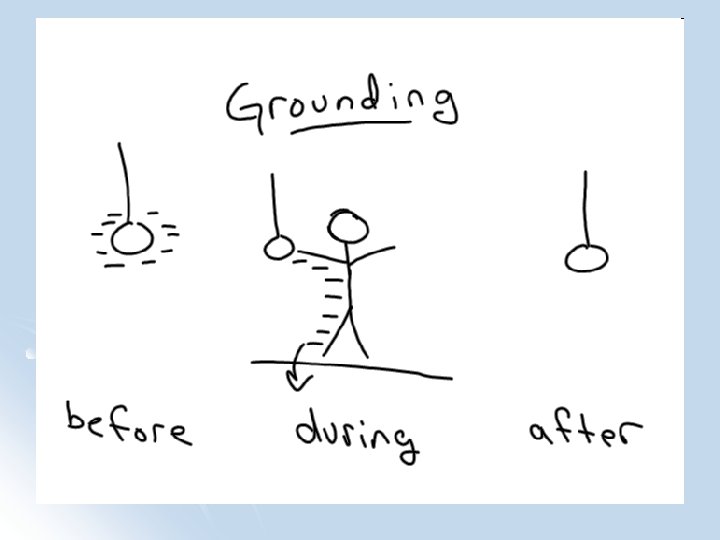
Grounding l Grounding: Allowing charge to move freely to the earth (often making the object neutral)

What Happened in the Lab?
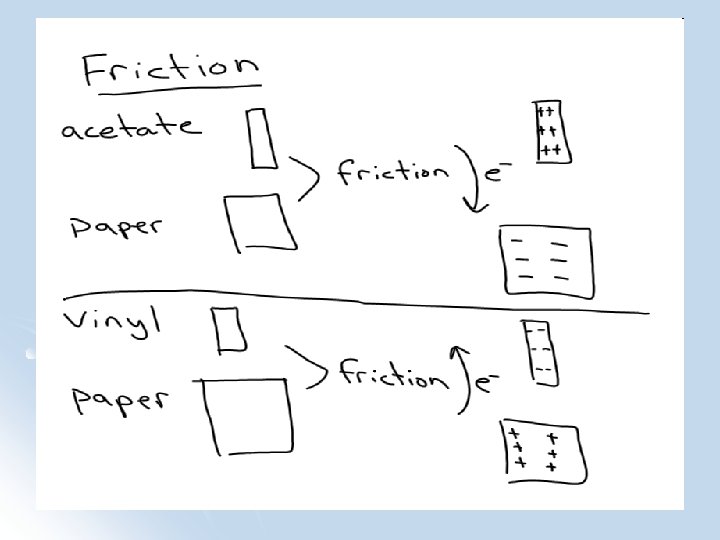
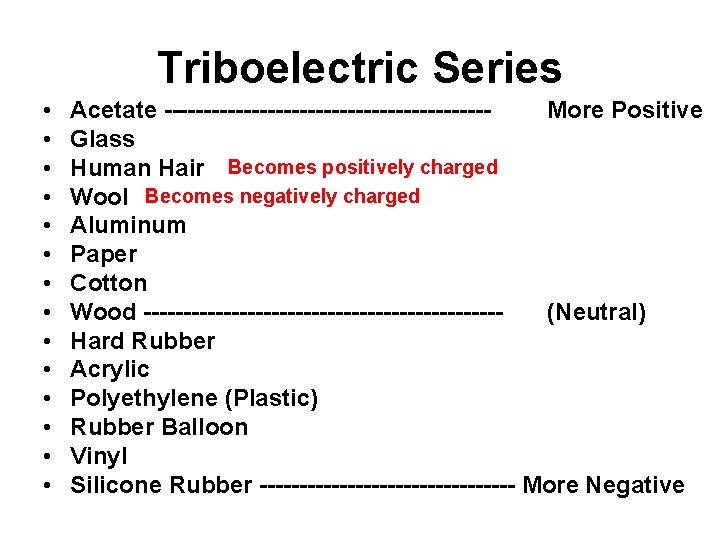
Triboelectric Series • • • • Acetate --------------------More Positive Glass Human Hair Becomes positively charged Wool Becomes negatively charged Aluminum Paper Cotton Wood ----------------------(Neutral) Hard Rubber Acrylic Polyethylene (Plastic) Rubber Balloon Vinyl Silicone Rubber ---------------- More Negative

Grounding l Grounding: Allowing charge to move freely to the earth (often making the object neutral)

Coulomb's Law
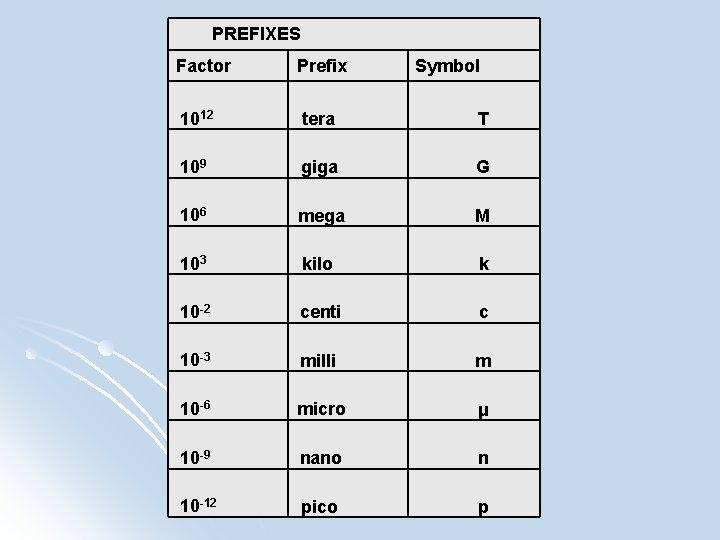
PREFIXES Factor Prefix Symbol 1012 tera T 109 giga G 106 mega M 103 kilo k 10 -2 centi c 10 -3 milli m 10 -6 micro μ 10 -9 nano n 10 -12 pico p

Example l If there is an electrical force of 0. 0345 N between two charges of -632 n. C what is there separation distance?

Homework l Electrostatic WS due Wed. 4/15/15

Circuit Wrap-Up
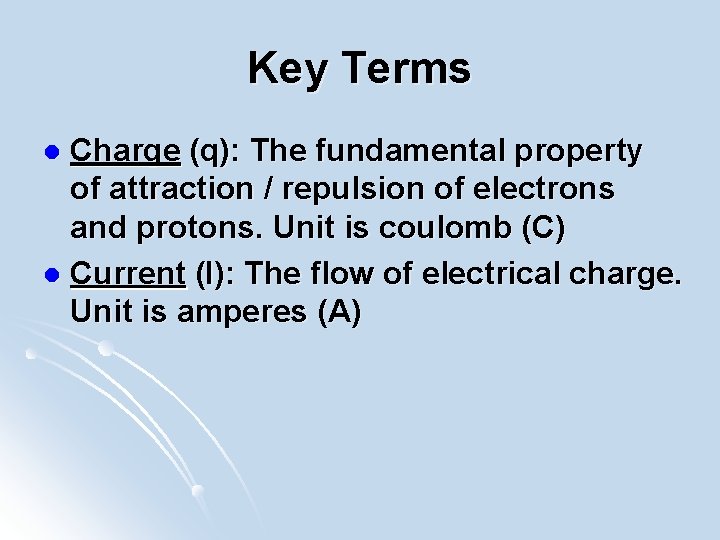
Key Terms Charge (q): The fundamental property of attraction / repulsion of electrons and protons. Unit is coulomb (C) l Current (I): The flow of electrical charge. Unit is amperes (A) l
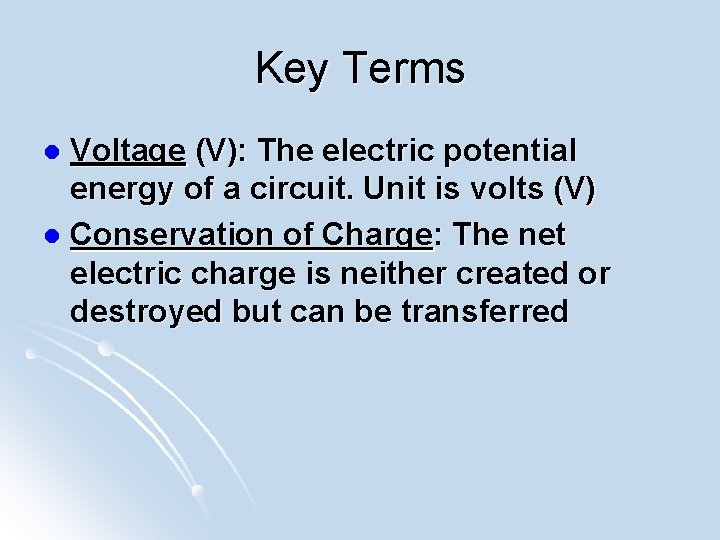
Key Terms Voltage (V): The electric potential energy of a circuit. Unit is volts (V) l Conservation of Charge: The net electric charge is neither created or destroyed but can be transferred l
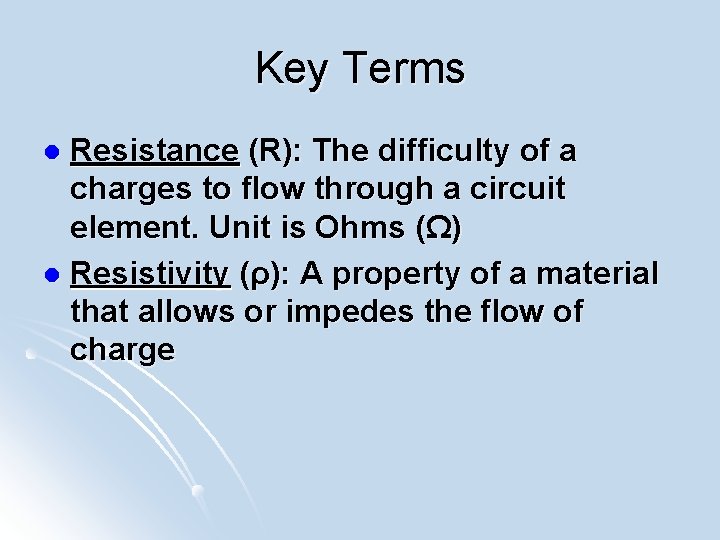
Key Terms Resistance (R): The difficulty of a charges to flow through a circuit element. Unit is Ohms (Ω) l Resistivity (ρ): A property of a material that allows or impedes the flow of charge l
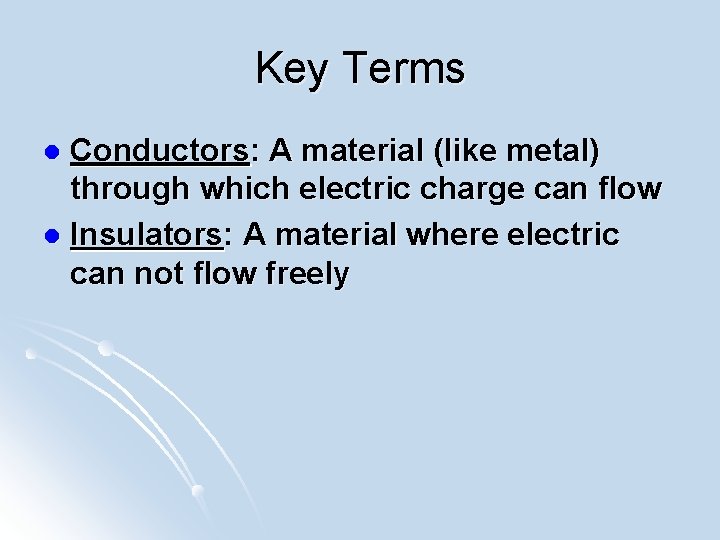
Key Terms Conductors: A material (like metal) through which electric charge can flow l Insulators: A material where electric can not flow freely l
Key Terms Series Circuit: Resistors connected in a single path l Parallel Circuit: Resistors are connected to the same two points of a circuit, so that any single resistor completes the circuit independently l
Conditions for a Circuit There must be a closed conducting path that extends from the positive terminal to the negative terminal. l There must be an electric potential difference across the two ends of the circuit. l
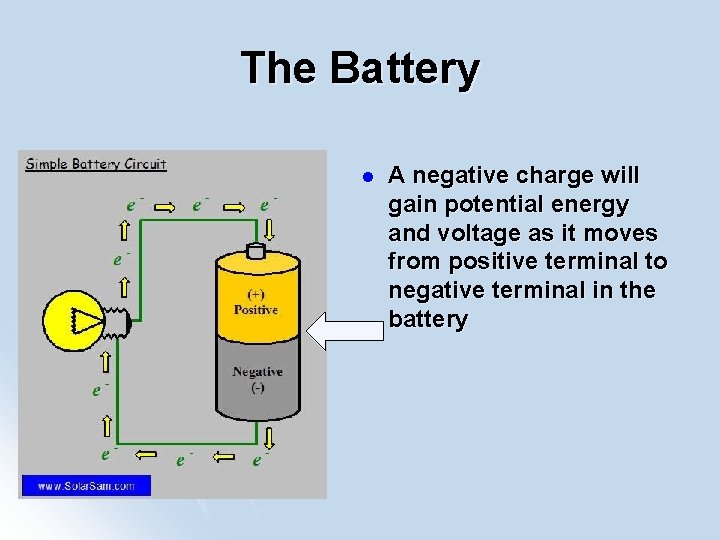
The Battery l A negative charge will gain potential energy and voltage as it moves from positive terminal to negative terminal in the battery
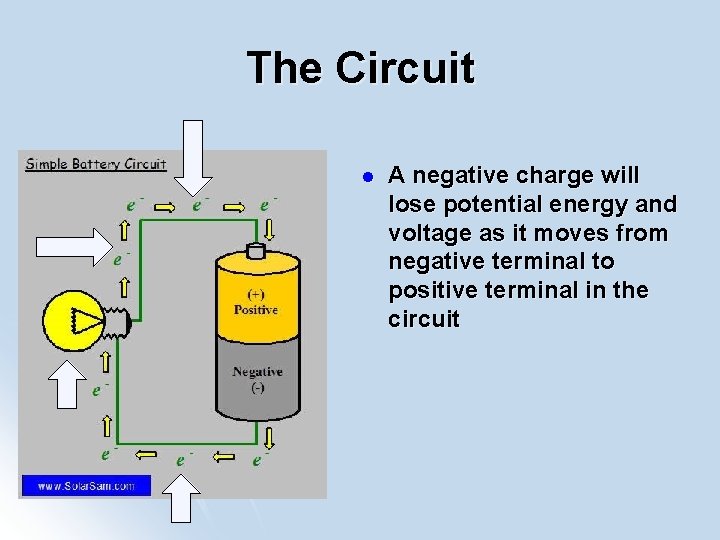
The Circuit l A negative charge will lose potential energy and voltage as it moves from negative terminal to positive terminal in the circuit
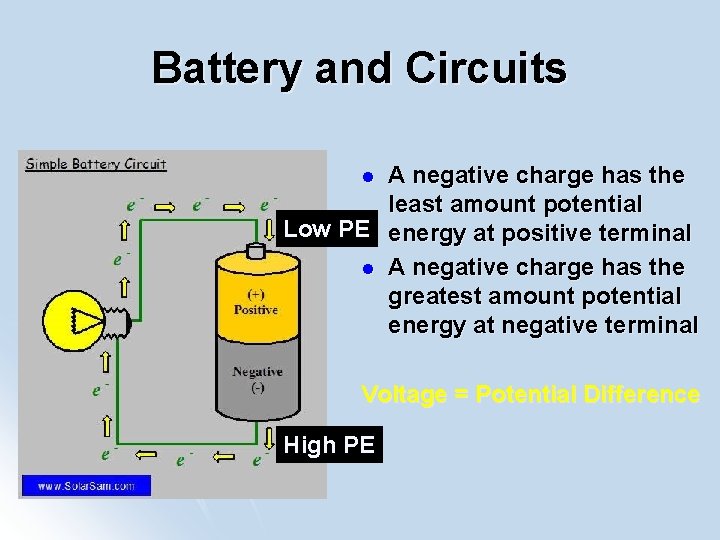
Battery and Circuits A negative charge has the least amount potential Low PE energy at positive terminal l A negative charge has the greatest amount potential energy at negative terminal l Voltage = Potential Difference High PE
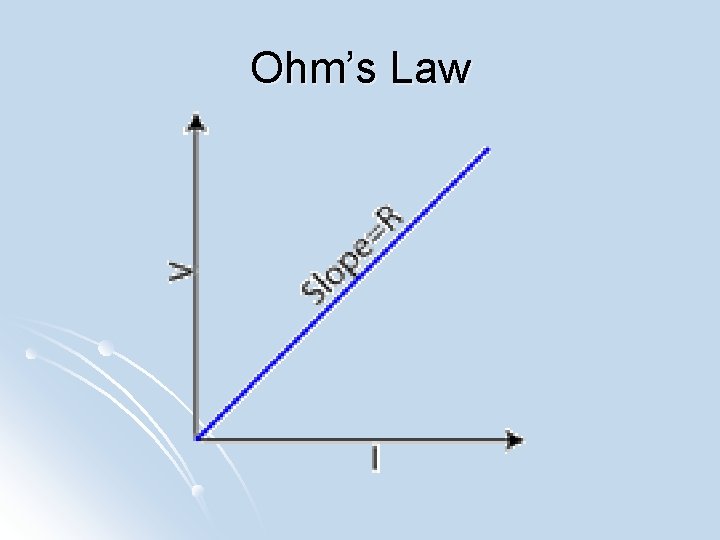
Ohm’s Law l Ohm's law: states that the current through a conductor between two points is directly proportional to the potential difference across the two points (linear relationship)
Ohm’s Law
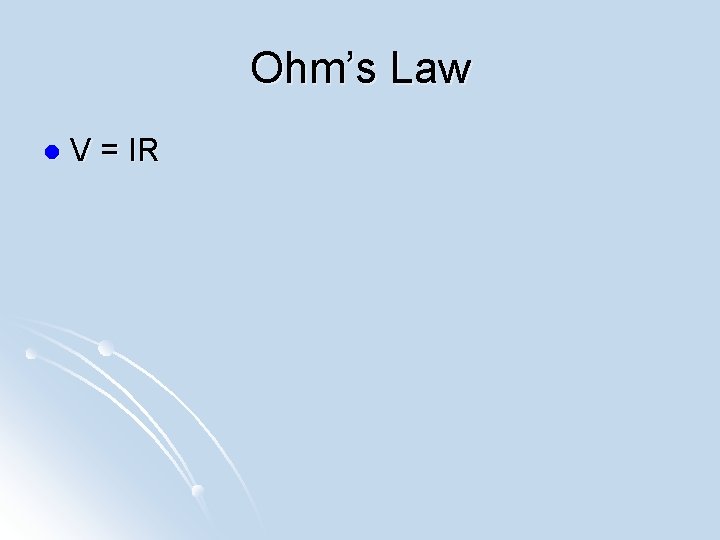
Ohm’s Law l V = IR
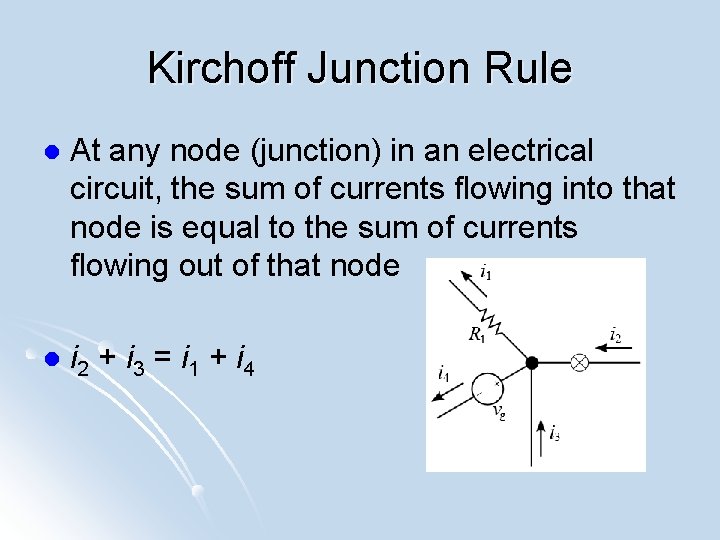
Kirchoff Junction Rule l At any node (junction) in an electrical circuit, the sum of currents flowing into that node is equal to the sum of currents flowing out of that node l i 2 + i 3 = i 1 + i 4
Kirchoff Loop Rule The directed sum of the electrical potential differences (voltage) around any closed network is zero l The energy of circuit has to obey the conservation of energy l
- Slides: 42