Electrontransfer reactions are called oxidationreduction reactions or redox



- Slides: 12


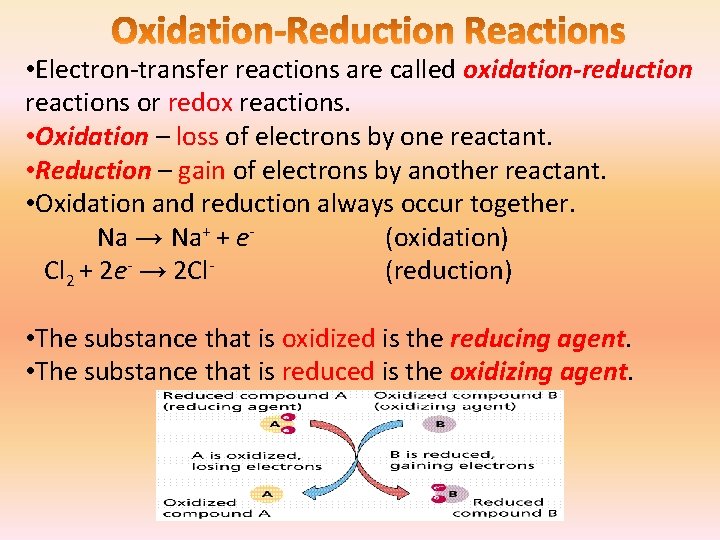
• Electron-transfer reactions are called oxidation-reduction reactions or redox reactions. • Oxidation – loss of electrons by one reactant. • Reduction – gain of electrons by another reactant. • Oxidation and reduction always occur together. Na → Na+ + e(oxidation) Cl 2 + 2 e- → 2 Cl(reduction) • The substance that is oxidized is the reducing agent. • The substance that is reduced is the oxidizing agent.

Oxidation Is Losing e- Reduction Is Gaining e- = OIL RIG

• Oxidation numbers provide a way to keep tabs on electron transfers. • Oxidation numbers – the sign is written before the number. • Electrical charges – the sign is written after the number. • Na ion has a charge of 1+ and an oxidation number of +1. • Since not all redox reactions produce ionic products, we can redefine redox reactions as chemical reactions in which changes in oxidation numbers occur.

Assignment of Oxidation Numbers Rules for Assigning Oxidation Numbers 1. The oxidation number of any free element (e. g. , O 2, Ag, etc. ) is zero, regardless of how complex its molecules may be. 2. The oxidation number for any simple, monatomic ion (e. g. , Na+ or Cl-) is equal to the charge on the ion. 3. The sum of all the oxidation numbers of the atoms in a molecule or polyatomic ion must equal the charge on the particle. 4. In its compounds, fluorine has an oxidation number of -1. 5. In its compounds, hydrogen has an oxidation number of +1. 6. In its compounds, oxygen has an oxidation number of -2.

• In binary ionic compounds with metals, the nonmetals have oxidation numbers equal to the charge on their anions. • Example: Assigning Oxidation Numbers • Molybdenum disulfide, Mo. S 2, has a structure that allows it to be used as a dry lubricant, much like graphite. What are the oxidation numbers of the atoms in Mo. S 2? • Solution: Binary ionic compound: S (2 atoms) x (-2) = -4 (Rule 2) Mo (1 atom) x (x) = x _________________ Sum = 0 (Rule 3) The value of x must be +4 for the sum to be zero. Therefore, Mo = +4 and S = -2

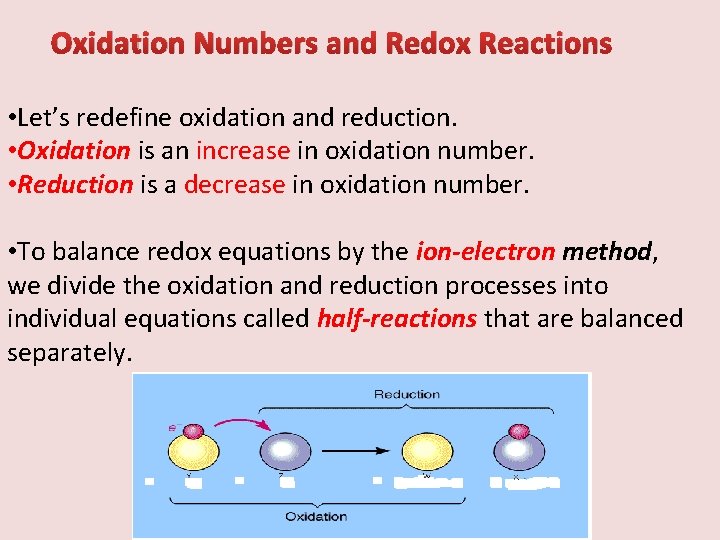
Oxidation Numbers and Redox Reactions • Let’s redefine oxidation and reduction. • Oxidation is an increase in oxidation number. • Reduction is a decrease in oxidation number. • To balance redox equations by the ion-electron method, we divide the oxidation and reduction processes into individual equations called half-reactions that are balanced separately.

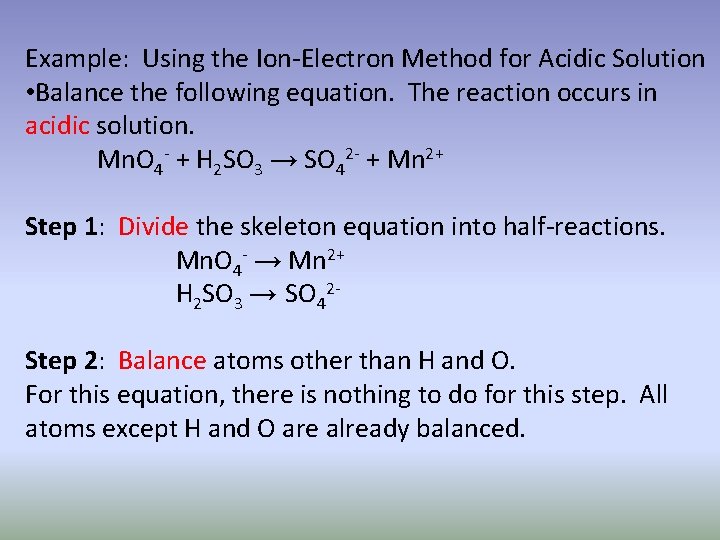
Example: Using the Ion-Electron Method for Acidic Solution • Balance the following equation. The reaction occurs in acidic solution. Mn. O 4 - + H 2 SO 3 → SO 42 - + Mn 2+ Step 1: Divide the skeleton equation into half-reactions. Mn. O 4 - → Mn 2+ H 2 SO 3 → SO 42 Step 2: Balance atoms other than H and O. For this equation, there is nothing to do for this step. All atoms except H and O are already balanced.

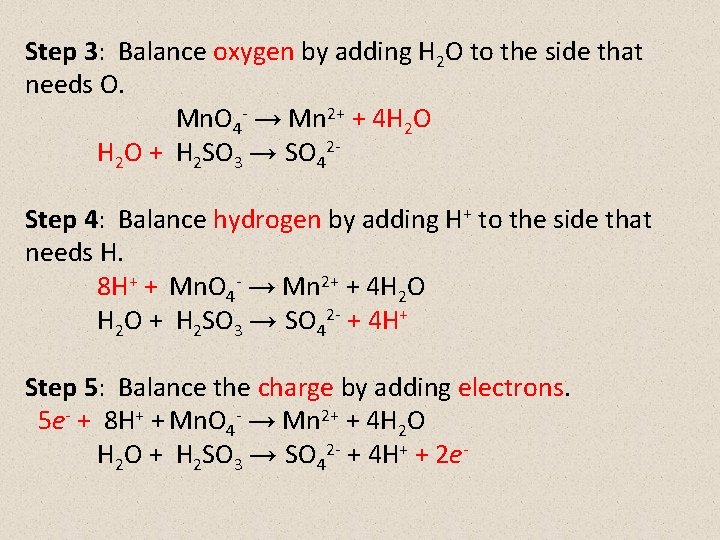
Step 3: Balance oxygen by adding H 2 O to the side that needs O. Mn. O 4 - → Mn 2+ + 4 H 2 O + H 2 SO 3 → SO 42 Step 4: Balance hydrogen by adding H+ to the side that needs H. 8 H+ + Mn. O 4 - → Mn 2+ + 4 H 2 O + H 2 SO 3 → SO 42 - + 4 H+ Step 5: Balance the charge by adding electrons. 5 e- + 8 H+ + Mn. O 4 - → Mn 2+ + 4 H 2 O + H 2 SO 3 → SO 42 - + 4 H+ + 2 e-

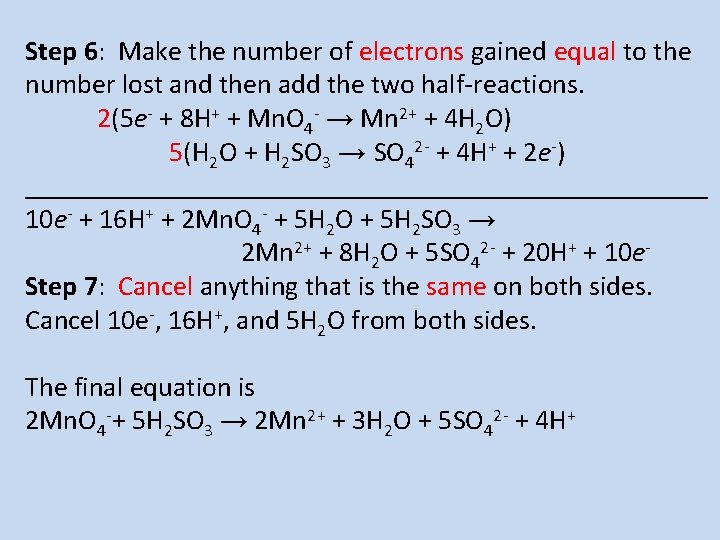
Step 6: Make the number of electrons gained equal to the number lost and then add the two half-reactions. 2(5 e- + 8 H+ + Mn. O 4 - → Mn 2+ + 4 H 2 O) 5(H 2 O + H 2 SO 3 → SO 42 - + 4 H+ + 2 e-) _________________________ 10 e- + 16 H+ + 2 Mn. O 4 - + 5 H 2 O + 5 H 2 SO 3 → 2 Mn 2+ + 8 H 2 O + 5 SO 42 - + 20 H+ + 10 e. Step 7: Cancel anything that is the same on both sides. Cancel 10 e-, 16 H+, and 5 H 2 O from both sides. The final equation is 2 Mn. O 4 -+ 5 H 2 SO 3 → 2 Mn 2+ + 3 H 2 O + 5 SO 42 - + 4 H+

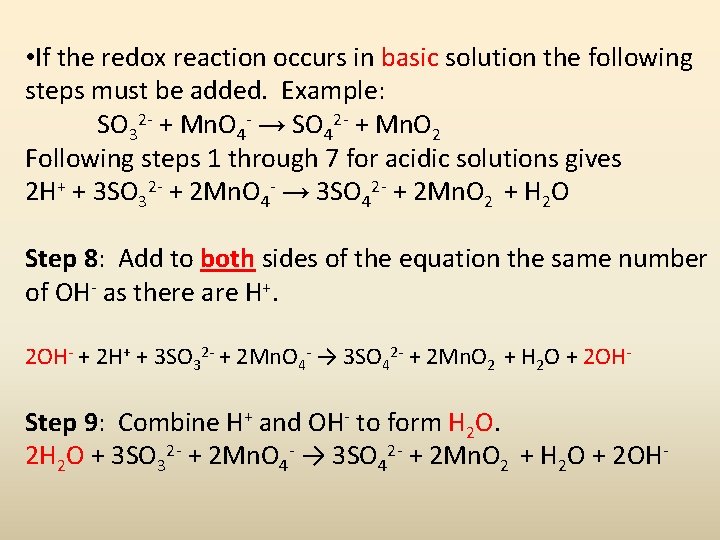
• If the redox reaction occurs in basic solution the following steps must be added. Example: SO 32 - + Mn. O 4 - → SO 42 - + Mn. O 2 Following steps 1 through 7 for acidic solutions gives 2 H+ + 3 SO 32 - + 2 Mn. O 4 - → 3 SO 42 - + 2 Mn. O 2 + H 2 O Step 8: Add to both sides of the equation the same number of OH- as there are H+. 2 OH- + 2 H+ + 3 SO 32 - + 2 Mn. O 4 - → 3 SO 42 - + 2 Mn. O 2 + H 2 O + 2 OH- Step 9: Combine H+ and OH- to form H 2 O. 2 H 2 O + 3 SO 32 - + 2 Mn. O 4 - → 3 SO 42 - + 2 Mn. O 2 + H 2 O + 2 OH-

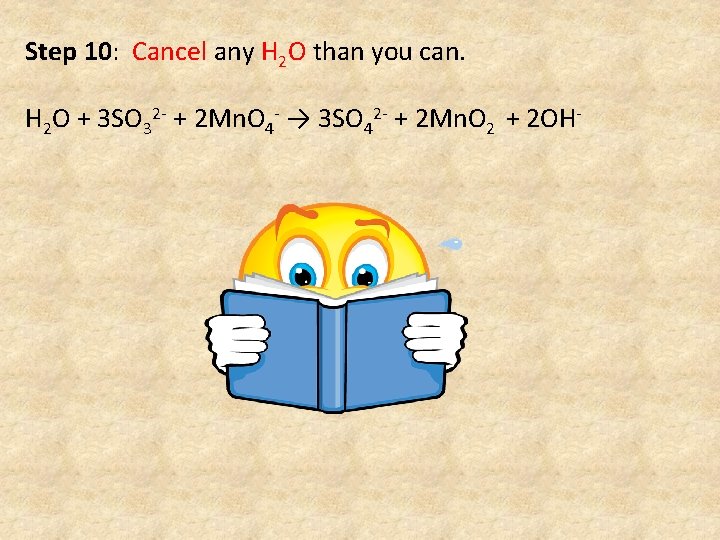
Step 10: Cancel any H 2 O than you can. H 2 O + 3 SO 32 - + 2 Mn. O 4 - → 3 SO 42 - + 2 Mn. O 2 + 2 OH-