Electrons and accelerators About yellow amber a winged

Electrons and accelerators About yellow amber, a winged god, purple haze and relativity K. Cornelis
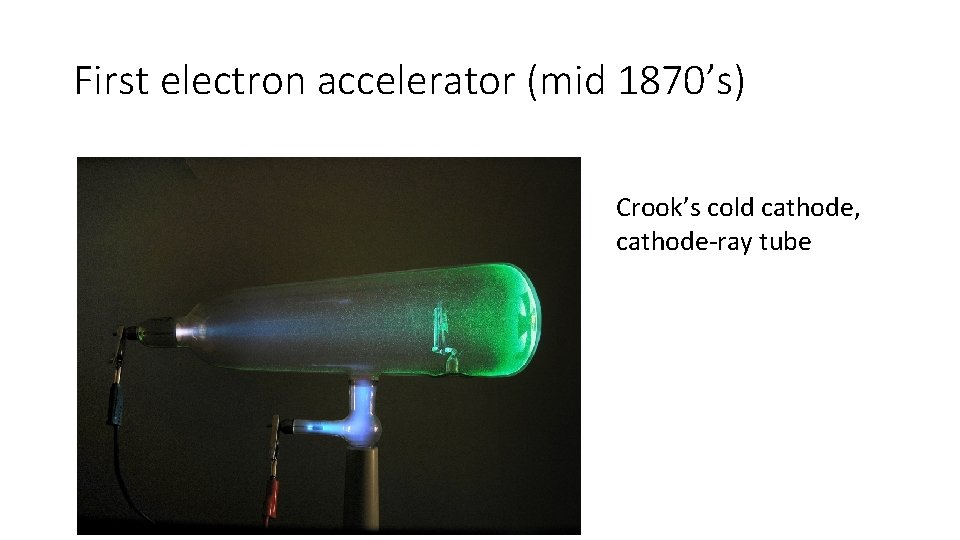
First electron accelerator (mid 1870’s) Crook’s cold cathode, cathode-ray tube

Evolution of gas discharge tubes ‘Purple haze’. Electric discharge in bad vacuum 1857 The development of this tubes and , finally, the revealing of electrons was possible thanks to the development of 2 new techniques : • Vacuum tight metal feedthroughs in glass Better vacuum revealed so called cathode rays 1878 • Vacuum pumps (better then 10 -3)

The neon tube was invented in 1857 by a glassblower (Heinrich Geissler)
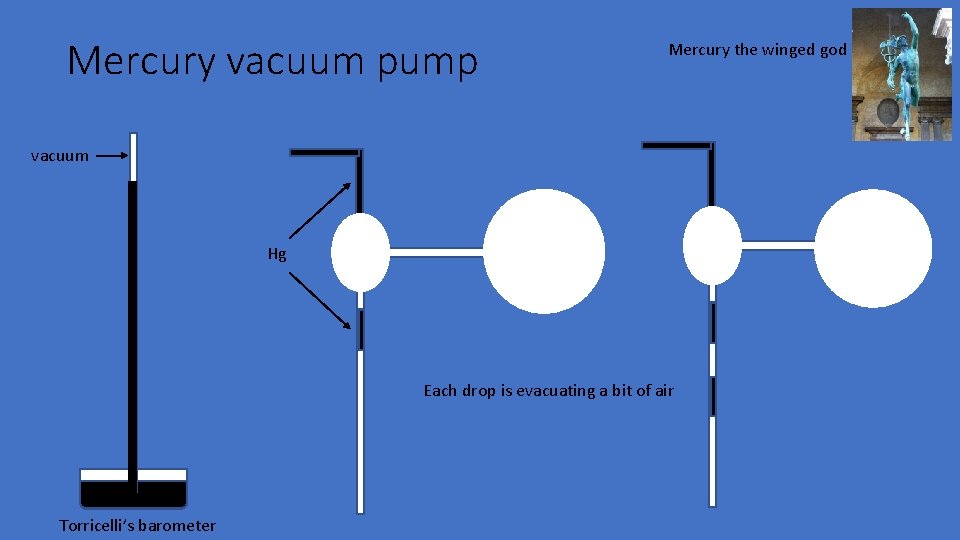
Mercury vacuum pump Mercury the winged god vacuum Hg Each drop is evacuating a bit of air Torricelli’s barometer
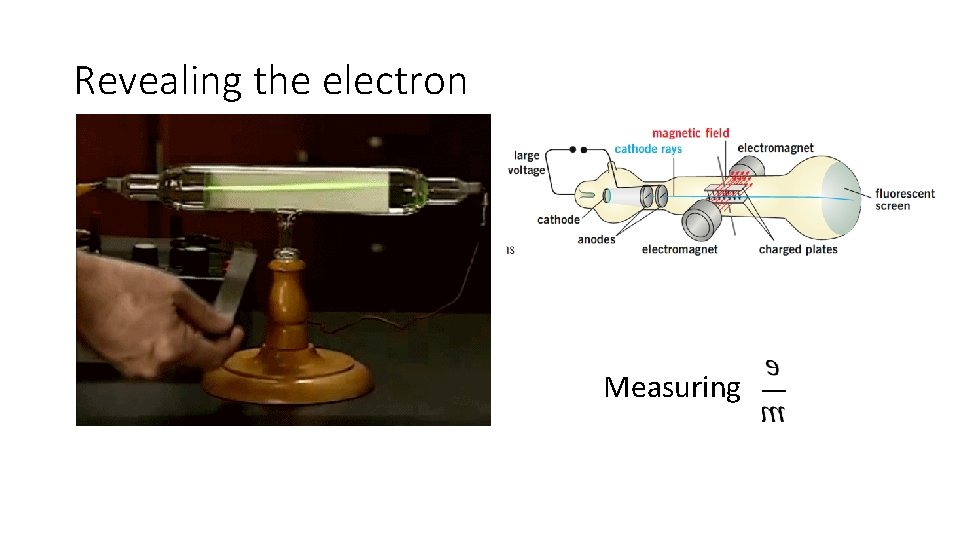
Revealing the electron Measuring
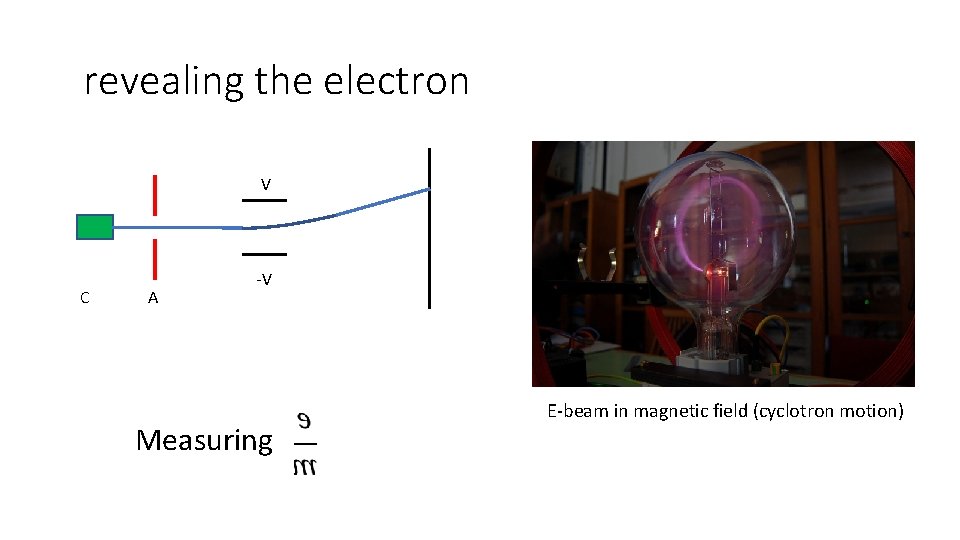
revealing the electron V C A -V Measuring E-beam in magnetic field (cyclotron motion)

The electron uncovered • After some years fooling around with cathode ray’s it became clear that it consisted of negative particles, with a mass at least 1000 times smaller then the hydrogen atom. (1997 J. J. Thomson et al. ) • At first the particles were named ‘corpuscules’, later they were baptised ‘electrons’ (Greek electros (elektros) which means yellow amber). They were identified as the carriers of electric current. • The charge (and hence the mass) could be determined from electrolysis experiments and the Avogadro number. • Later, the oil drop experiment was performed by Robert A. Millikan and Harvey Fletcher in 1909 to measure the elementary charge more precisely.

First estimation of Avogadro’s number
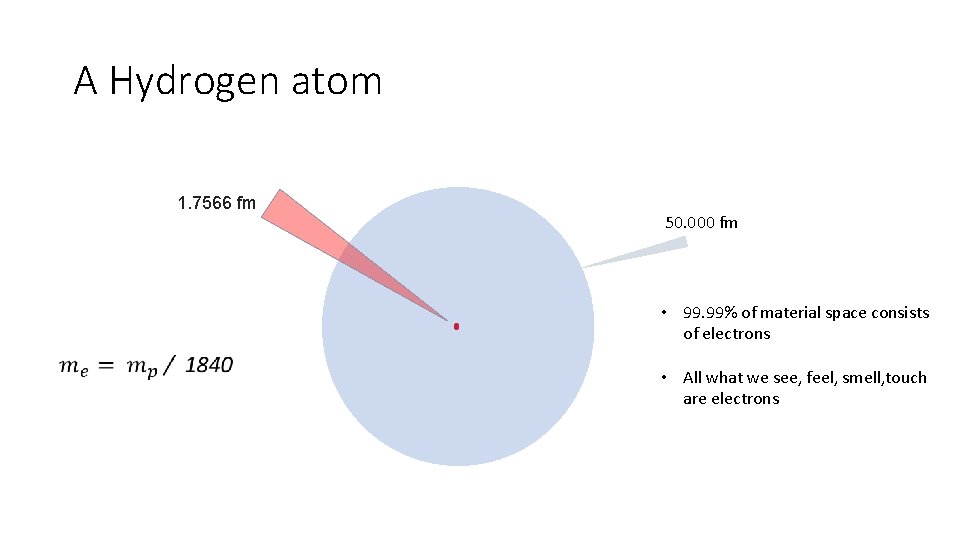
A Hydrogen atom 1. 7566 fm 50. 000 fm • 99. 99% of material space consists of electrons • All what we see, feel, smell, touch are electrons
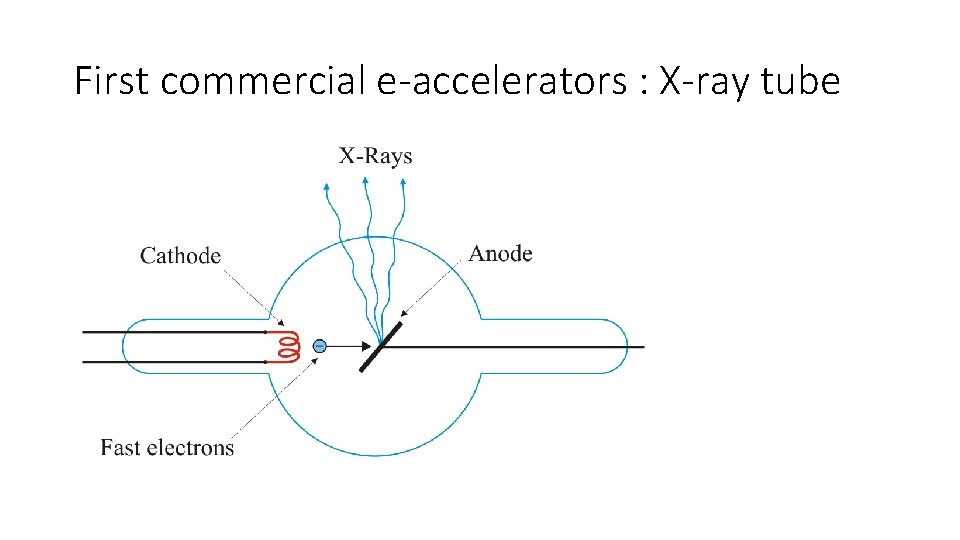
First commercial e-accelerators : X-ray tube

First commercial e-accelerators : Cathode ray tubes
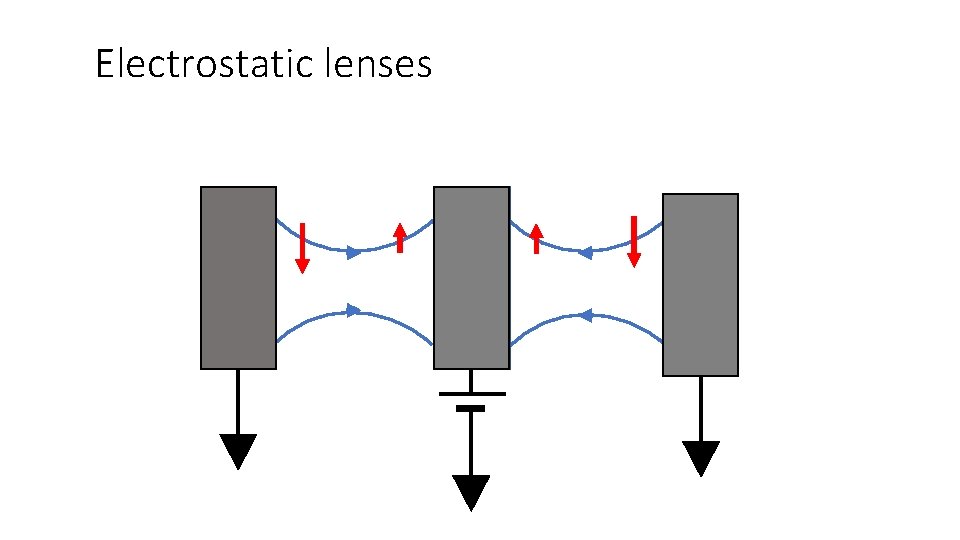
Electrostatic lenses
Television tubes Use of magnetic coils for deflection and focus
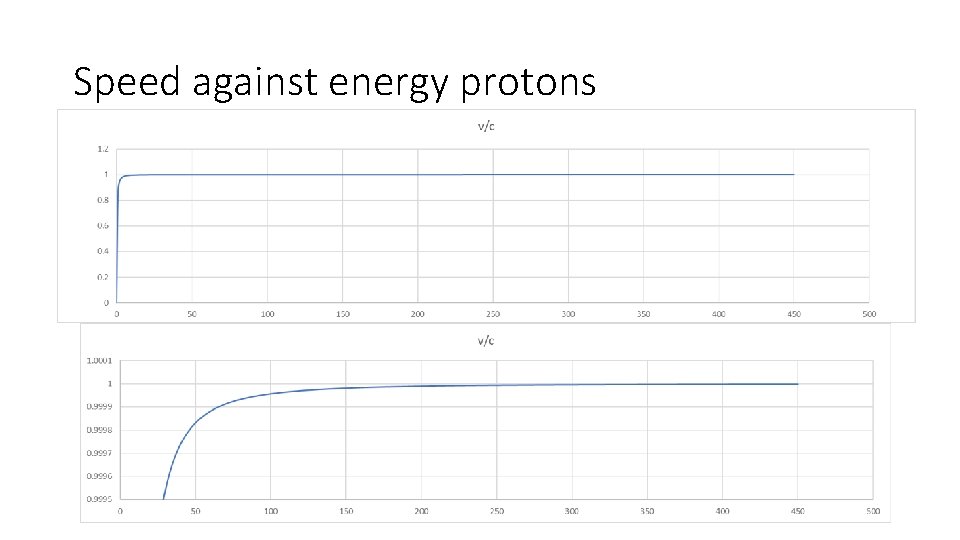
Speed against energy protons
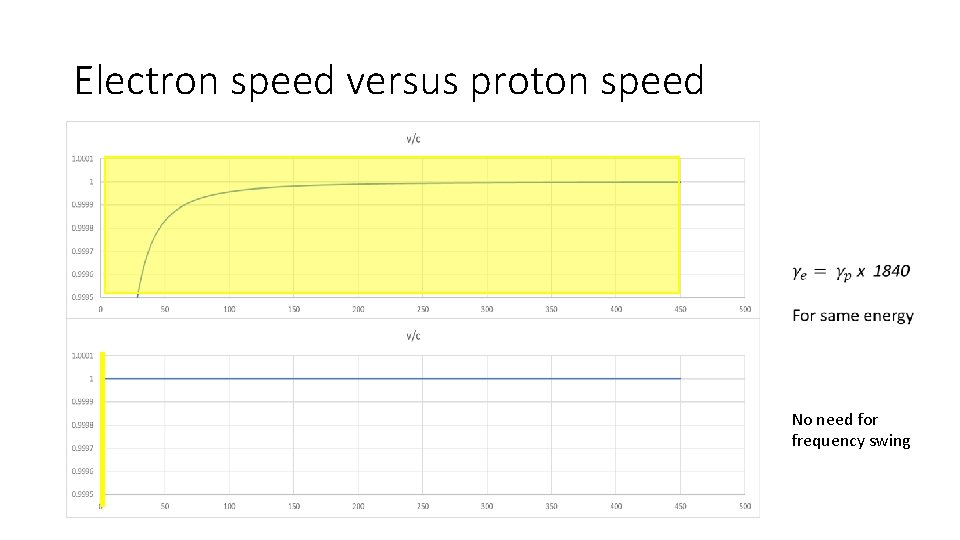
Electron speed versus proton speed No need for frequency swing

Relativity and it’s consequences (Lorentz transforms) 1893 ‘When in the limit v = c, the increase in mass is infinite, thus a charged sphere moving with the velocity of light behaves as if its mass were infinite, its velocity therefore will remain constant, in other words it is impossible to increase the velocity of a charged body beyond that of light. ’ Lorentz contraction Lorentz factor
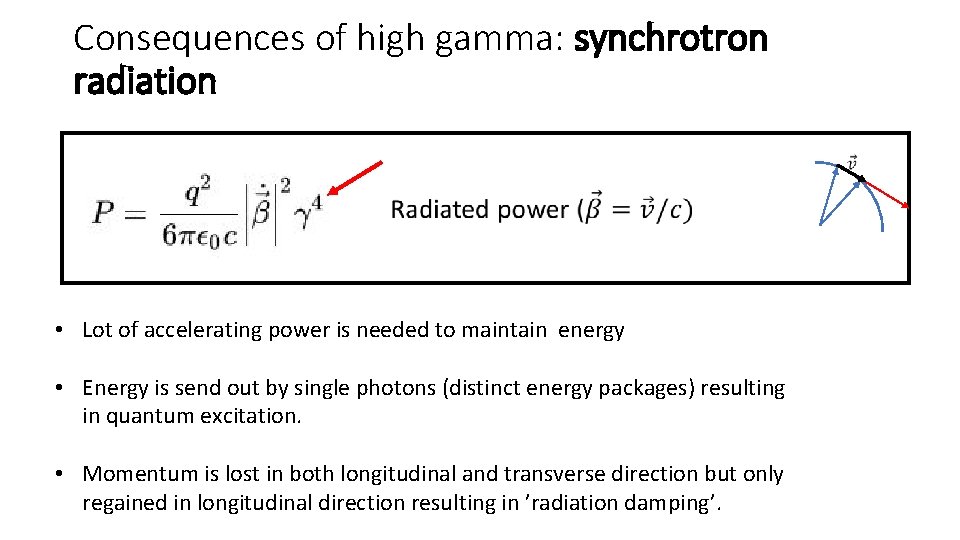
Consequences of high gamma: synchrotron radiation • Lot of accelerating power is needed to maintain energy • Energy is send out by single photons (distinct energy packages) resulting in quantum excitation. • Momentum is lost in both longitudinal and transverse direction but only regained in longitudinal direction resulting in ’radiation damping’.
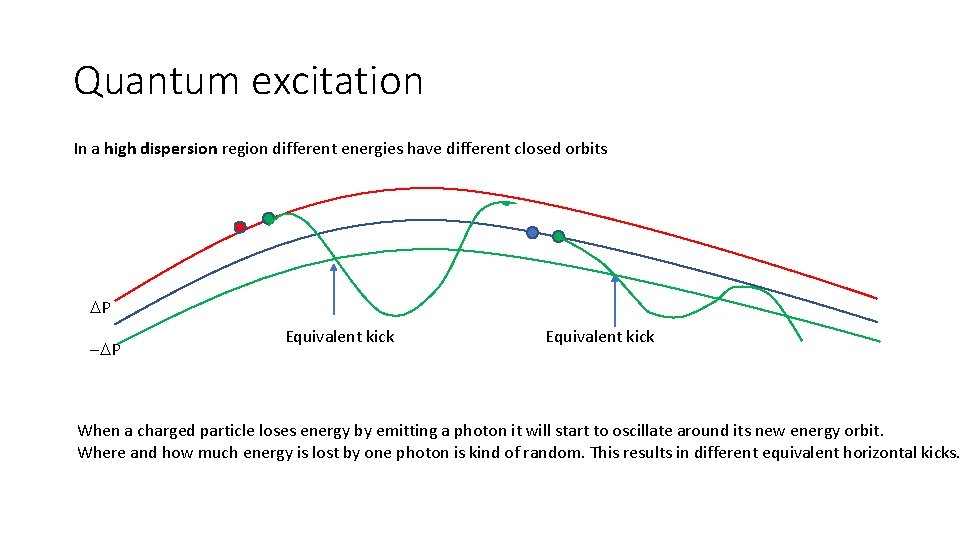
Quantum excitation In a high dispersion region different energies have different closed orbits DP -DP Equivalent kick When a charged particle loses energy by emitting a photon it will start to oscillate around its new energy orbit. Where and how much energy is lost by one photon is kind of random. This results in different equivalent horizontal kicks.
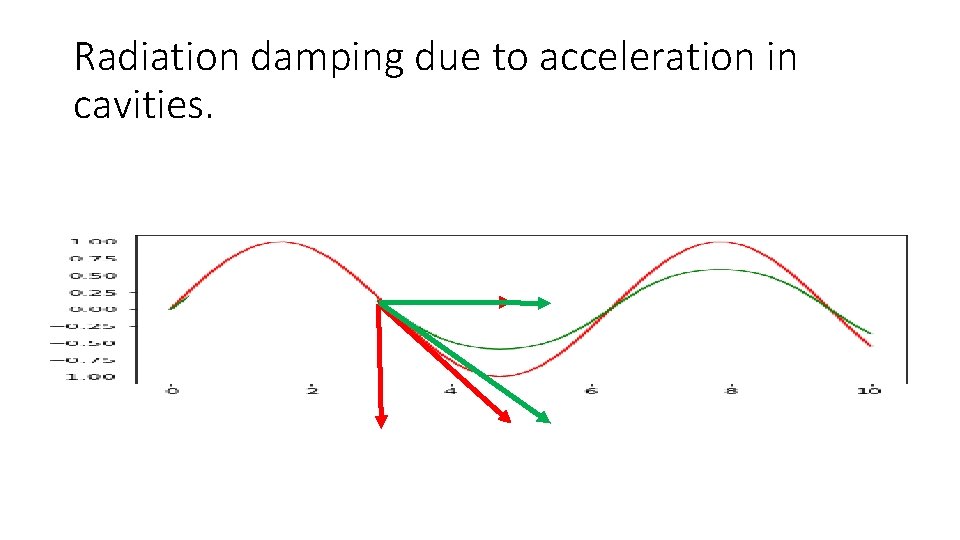
Radiation damping due to acceleration in cavities.

Quantum excitation and radiation damping define the shape of e-beams • Quantum excitation takes place in the horizontal plane (needs dispersion) whereas radiation damping takes place in both planes. • In a perfect accelerator the beam just be a horizontal stripe. • In a real accelerator there is always some vertical dispersion and coupling creating a small vertical beam size. • Time profile = space profile (IMPORTANCE OF APPERTURE)
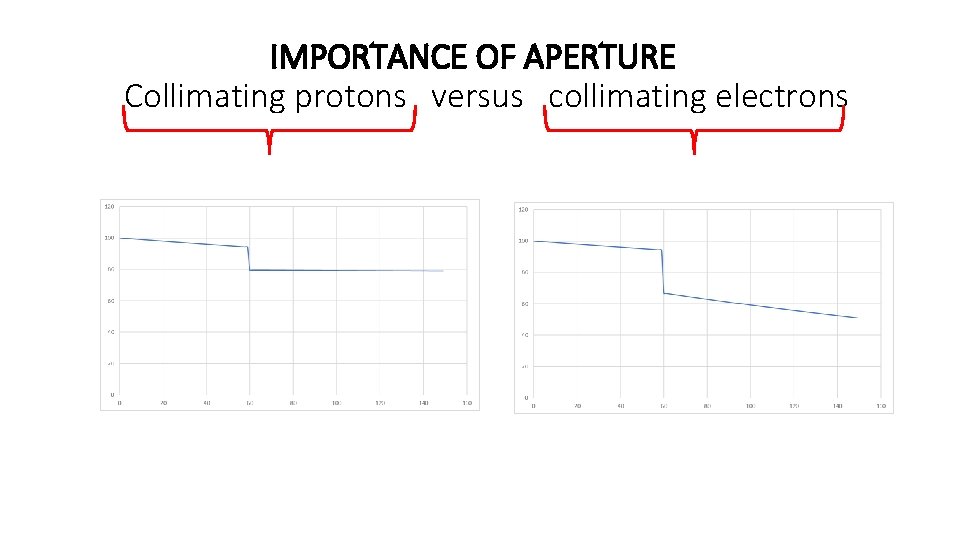
IMPORTANCE OF APERTURE Collimating protons versus collimating electrons
IMPORTANCE OF APERTURE • Need for very high RF voltage to increase momentum acceptance • Qs very high • Synchro-betatron resonances (Avoid multiples of Qs)
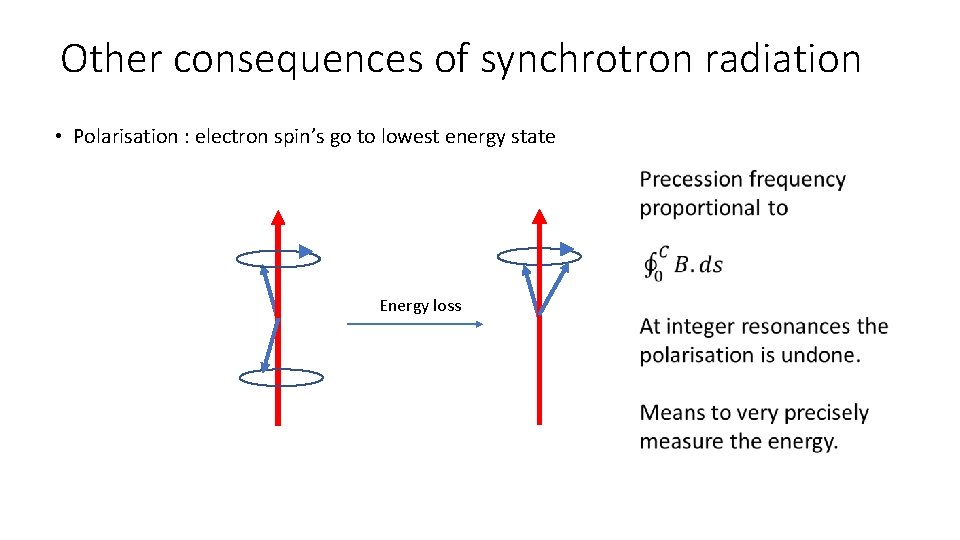
Other consequences of synchrotron radiation • Polarisation : electron spin’s go to lowest energy state Energy loss

RESUMEE • With the first accelerators came the identification of electrons, which was recognised as the elementary charge and carrier of electrical current. • The electron was the first elementary particle to be identified and this was in the 19 th century. • e-accelerators not only served the progress of science, they provided very useful machinery. • X-rays, Oscilloscopes, radar screens, TV and computer screens, synchrotron light sources, medical accelerators, RF-tubes, klystrons, …

RESUMEE (suite) • Electrons reach very quickly the speed of light resulting in: • No problems with space charge and IBS. • Synchroton radiation • High RF voltages (high Qs) • Radiation damping and quantum excitation. • Polarisation (energy calibration) • NB: The interest for physics is that electron collisions have a well defined energy.
- Slides: 27