Electronic Structure of Atoms The Quantum Mechanical Model









- Slides: 23

Electronic Structure of Atoms - The Quantum Mechanical Model

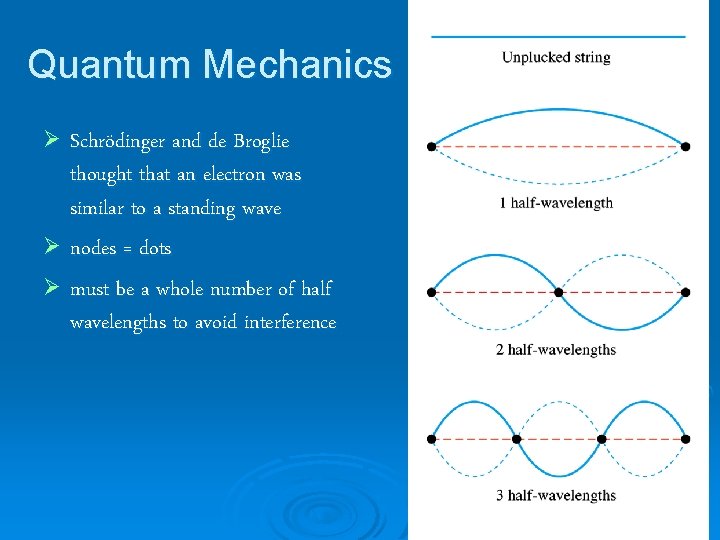
Quantum Mechanics Ø Schrödinger and de Broglie thought that an electron was similar to a standing wave Ø nodes = dots Ø must be a whole number of half wavelengths to avoid interference

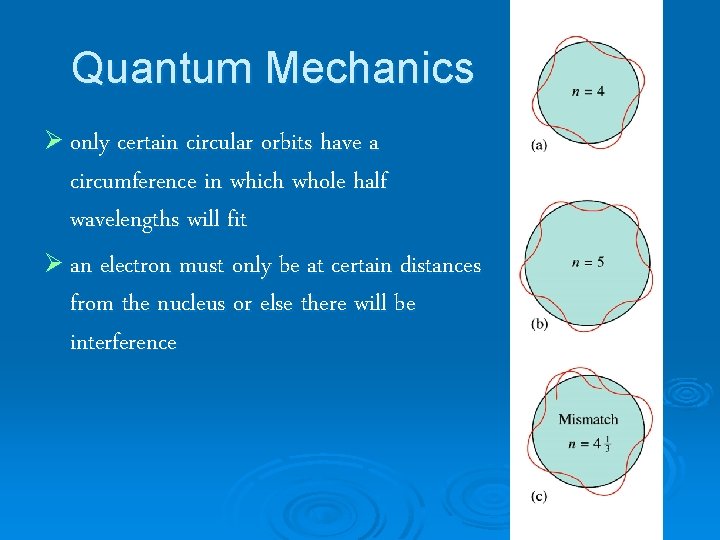
Quantum Mechanics Ø only certain circular orbits have a circumference in which whole half wavelengths will fit Ø an electron must only be at certain distances from the nucleus or else there will be interference

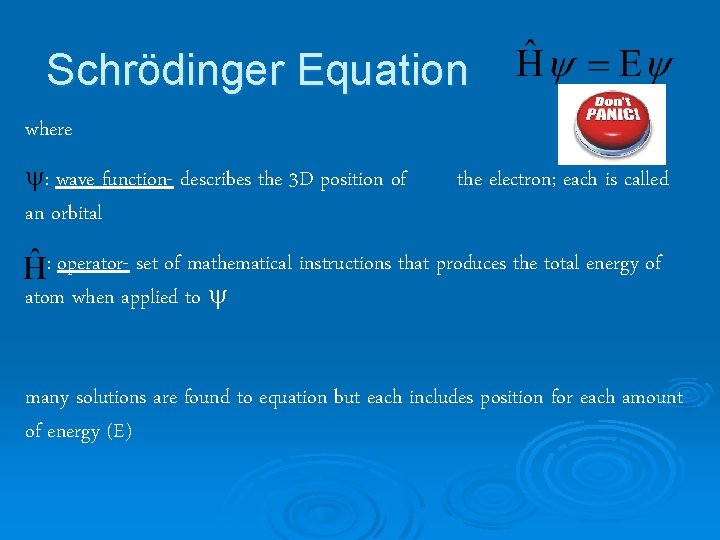
Schrödinger Equation where : wave function- describes the 3 D position of an orbital the electron; each is called : operator- set of mathematical instructions that produces the total energy of atom when applied to many solutions are found to equation but each includes position for each amount of energy (E)

Heisenberg Uncertainty Principle Ø we cannot know the exact position and momentum (motion) of the electron Ø as more is known about position, less is known about momentum Ø uncertainties are inversely proportional where ∆x: uncertainty in position ∆m : uncertainty in mometum Øminimum uncertainty is h/4

Meaning of Wave Function Ø the wave function itself does not have concrete meaning Ø the square of the wave function represents the probability of finding an electron at a certain point Ø easily represented as probability distribution where the deepness of color indicates the probability

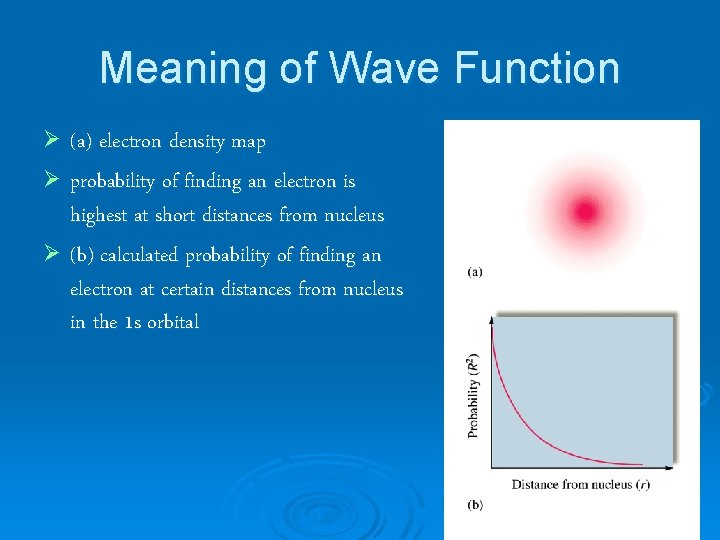
Meaning of Wave Function Ø (a) electron density map Ø probability of finding an electron is highest at short distances from nucleus Ø (b) calculated probability of finding an electron at certain distances from nucleus in the 1 s orbital

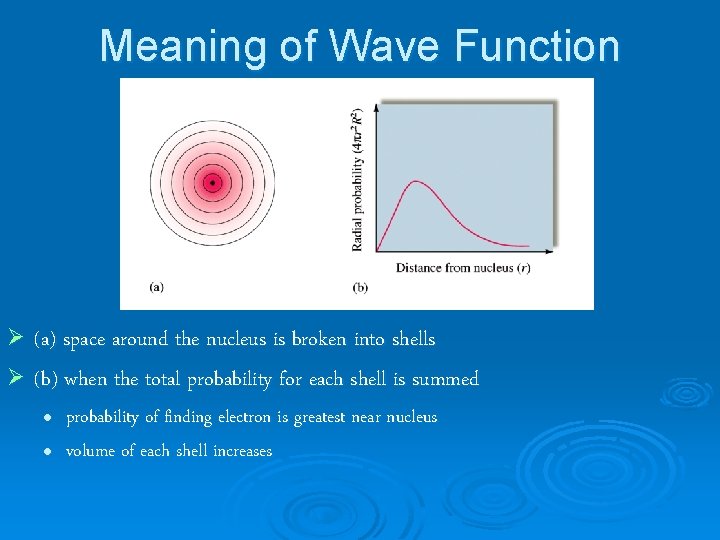
Meaning of Wave Function Ø (a) space around the nucleus is broken into shells Ø (b) when the total probability for each shell is summed l l probability of finding electron is greatest near nucleus volume of each shell increases

Electronic Structure of Atoms - Quantum Numbers - Orbital Shapes and Energies - Electron Spin and Pauli’s Principle

Quantum Numbers Ø specify the properties of atomic orbitals and of electrons in orbitals Ø the first three numbers come from the Schrödinger equation and describe: l l l main energy level shape orientation Ø 4 th describes state of electron

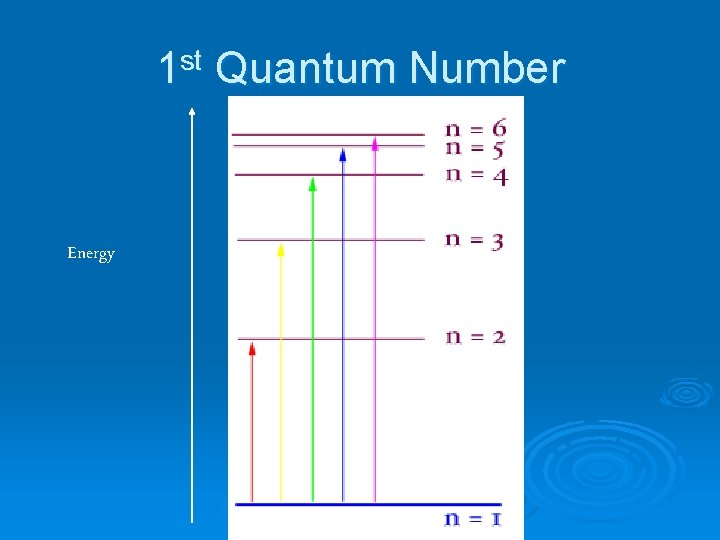
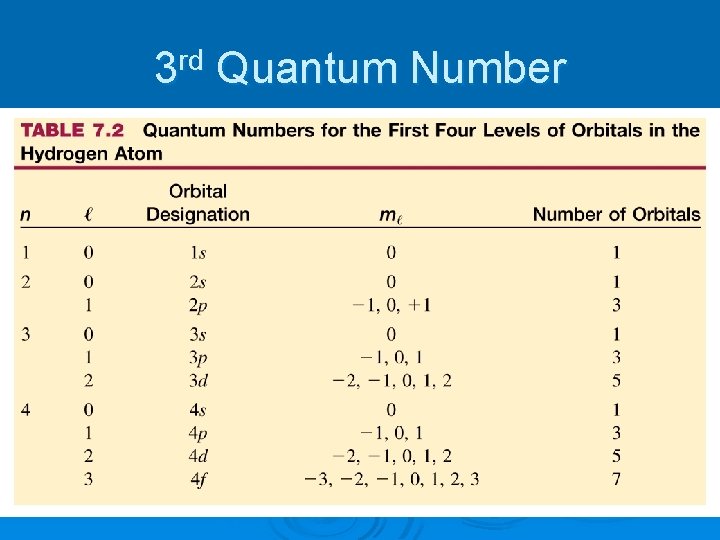
1 st Quantum Number Principal Quantum Number: n Ø main energy level (or shell) occupied by electron Ø values are all positive integers (1, 2, 3, …) Ø As n increases l l l size of orbital is larger electron has higher energy the electron’s average distance from the nucleus increases

1 st Quantum Number Energy

2 nd Quantum Number Angular Momentum Quantum Number: l Ø indicates the shape of the orbital (sublevel or subshell) Ø the number of possible shapes (or l values) for an energy level is equal to n Ø the possible values of l are 0 and all positive integers less than or equal to n - 1

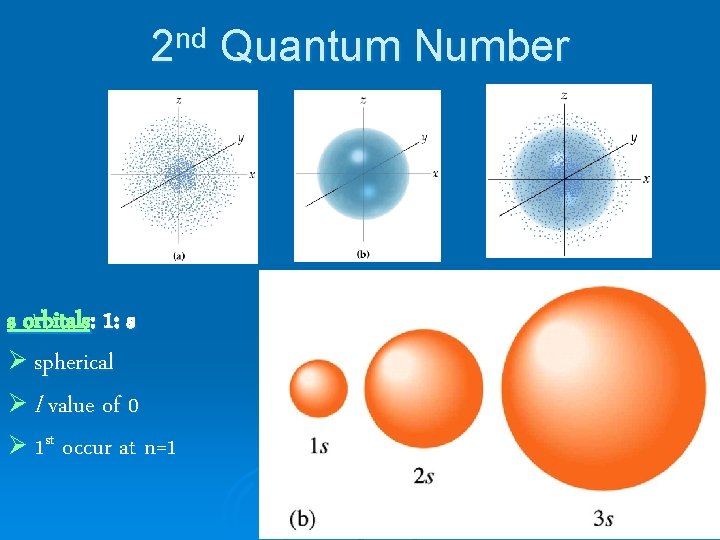
2 nd Quantum Number s orbitals: 1: s Ø spherical Ø l value of 0 Ø 1 st occur at n=1

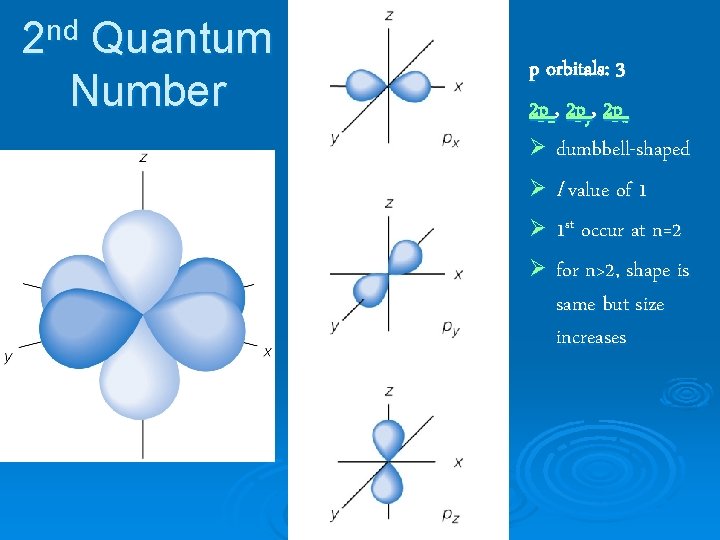
nd 2 Quantum Number p orbitals: 3 2 px, 2 py, 2 pz Ø dumbbell-shaped Ø l value of 1 Ø 1 st occur at n=2 Ø for n>2, shape is same but size increases

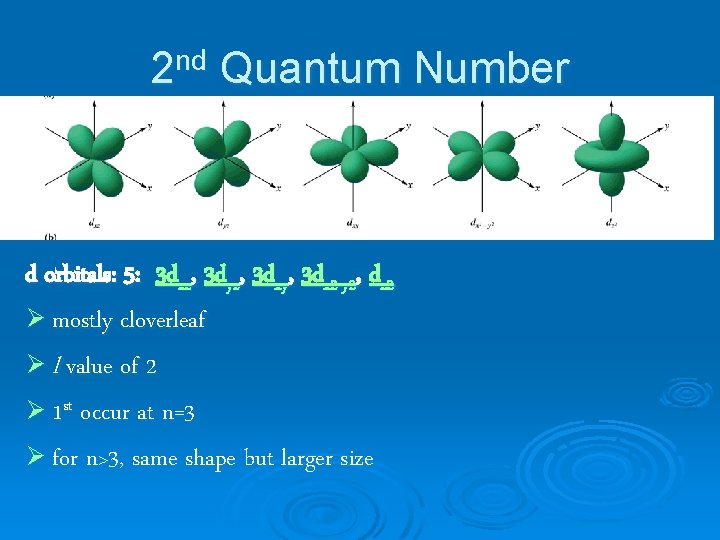
2 nd Quantum Number d orbitals: 5: 3 dxz, 3 dyz, 3 dxy, 3 dx 2 -y 2, dz 2 Ø mostly cloverleaf Ø l value of 2 Ø 1 st occur at n=3 Ø for n>3, same shape but larger size

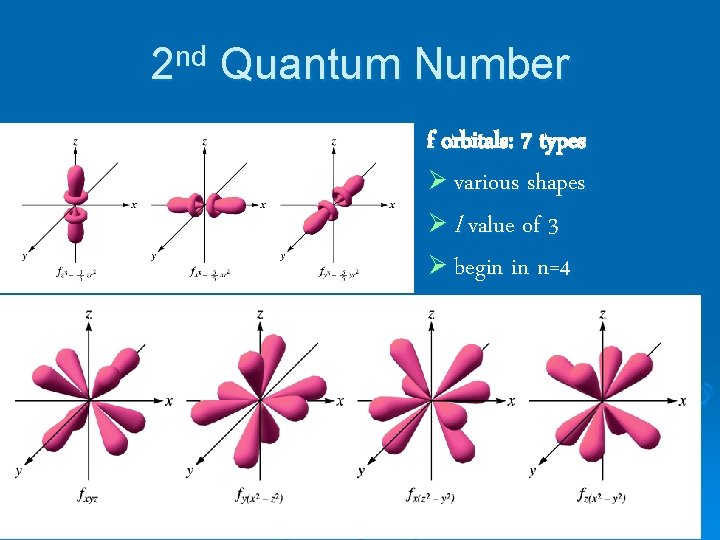
2 nd Quantum Number f orbitals: 7 types Ø various shapes Ø l value of 3 Ø begin in n=4

2 nd Quantum Number Ø other shapes can exist in energy levels as long as they follow the rules Ø g (l=4) starts in 5 with 9 orbitals Ø h (l=5) starts in 6 with 11 orbitals, etc Ø but no known elements have electrons in them at ground state

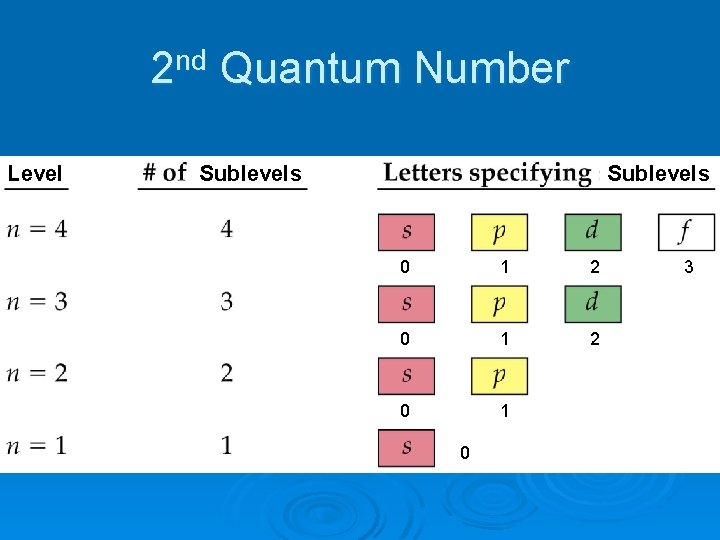
2 nd Quantum Number Level Sublevels 0 1 2 0 1 0 3

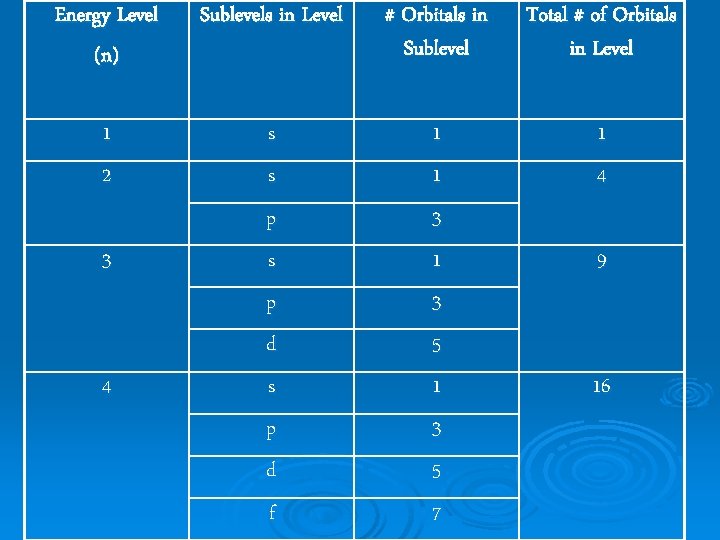
3 rd Quantum Number Magnetic Quantum Number: ml Ø indicates the orientation of an orbital around the nucleus Ø has values from +l -l Ø specifies the exact orbital that the electron is contained in Ø each orbital holds maximum of 2 electrons Ø total number of orbitals is equal to n 2 for an energy level Ø number of possible ml values for a certain subshell is equal to 2 l + 1

3 rd Quantum Number

Energy Level (n) Sublevels in Level # Orbitals in Sublevel Total # of Orbitals in Level 1 2 s s 1 1 1 4 3 p s 3 1 9 p d 3 5 s p 1 3 d f 5 7 4 16

4 th Quantum Number Spin Quantum Number: ms Ø indicates the spin state of the electron Ø only 2 possible directions Ø only 2 possible values: +½ and -½ Ø paired electrons must have opposite spins Ø maximum number of electrons in an energy level is 2 n 2