ELECTRONIC STRUCTURE Canvas lesson 03 Electronic Structure Todays









- Slides: 21

ELECTRONIC STRUCTURE Canvas lesson: “ 03 – Electronic Structure”

Today’s Lesson – The Key to Chemistry

Learning Goals 1. Draw a labelled diagram to that describes how electrons are arranged in an atom. 2. Draw the electronic configuration of the first 20 elements, using both diagrams and numbers. 3. Answer questions to describe the link between electron configuration and chemical reactivity.

Filling Electron Shells 2 important rules to remember: 1. Fill the electron shells in order, the first shell then the second etc. 2. The shells can only hold a certain number of electrons 1 st shell – maximum of 2 electrons. 2 nd shell – maximum of 8 electrons. 3 rd shell – maximum of 8 electrons. Draw a labelled diagram to that describes how electrons are arranged in an atom.

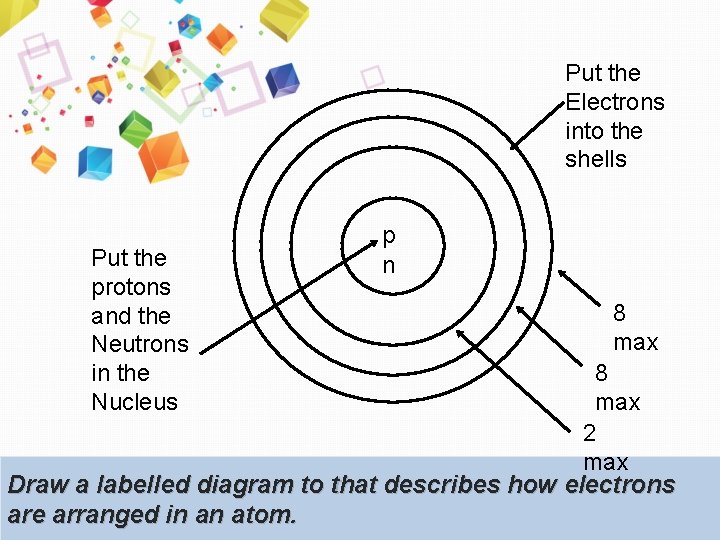
Put the Electrons into the shells Put the protons and the Neutrons in the Nucleus p n 8 max 2 max Draw a labelled diagram to that describes how electrons are arranged in an atom.

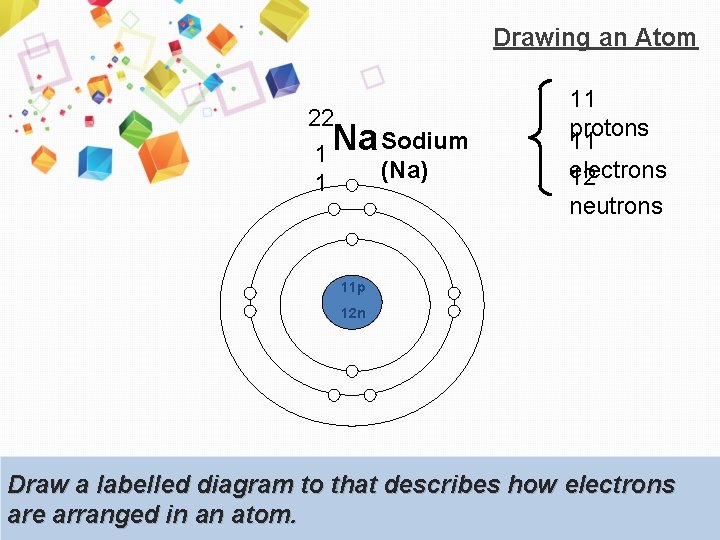
Drawing an Atom 22 1 1 Na Sodium (Na) 11 protons 11 electrons 12 neutrons 11 p 12 n Draw a labelled diagram to that describes how electrons are arranged in an atom.

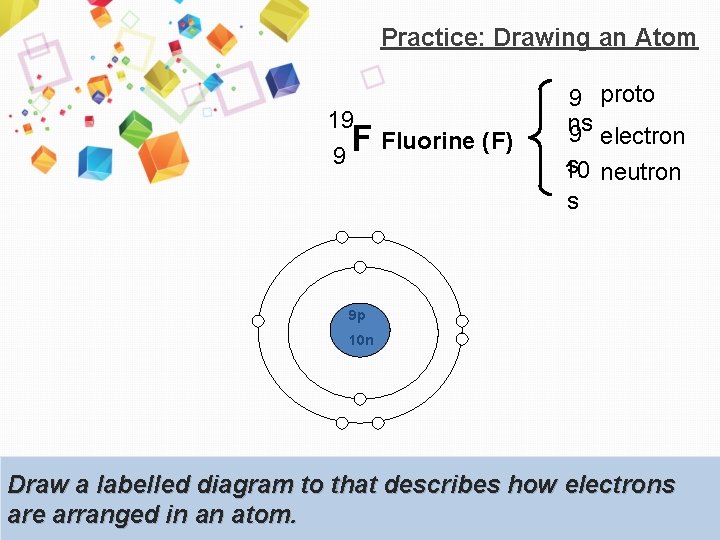
Practice: Drawing an Atom 19 9 F Fluorine (F) 9 proto ns 9 electron s neutron 10 s 9 p 10 n Draw a labelled diagram to that describes how electrons are arranged in an atom.

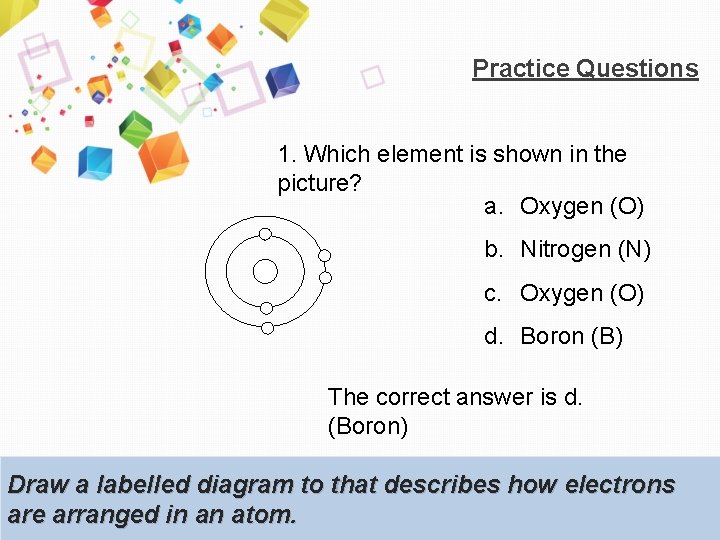
Practice Questions 1. Which element is shown in the picture? a. Oxygen (O) b. Nitrogen (N) c. Oxygen (O) d. Boron (B) The correct answer is d. (Boron) Draw a labelled diagram to that describes how electrons are arranged in an atom.

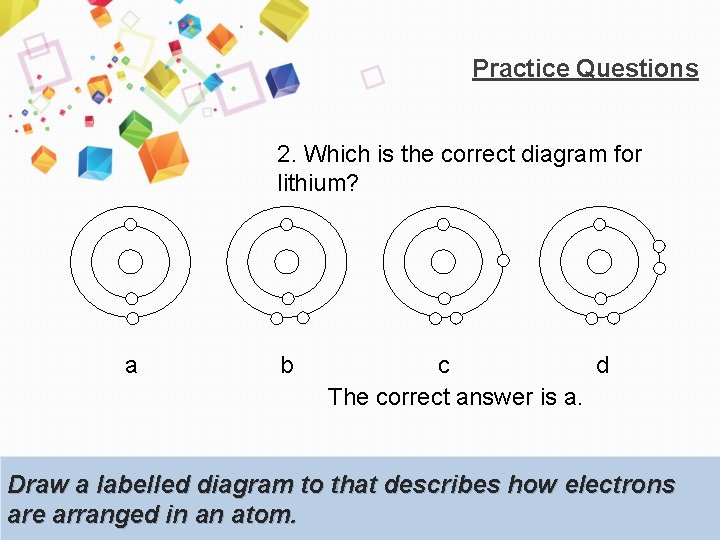
Practice Questions 2. Which is the correct diagram for lithium? a b c d The correct answer is a. Draw a labelled diagram to that describes how electrons are arranged in an atom.

Drawing Electronic Configurations Using the sheet provided complete the electronic configuration for the first 20 atoms. • Neon (Ne) has been done for you. • Answer the questions. • You have 15 minutes. Draw the electronic configuration of the first 20 elements, using both diagrams and numbers.

Writing Electronic Configuration As well as drawing an atom, the electronic configuration can be given in writing. For example: • Lithium: 2, 1 • Fluorine: 2, 7 Write the electronic configurations for the following: • Na, K, Cl, Ca, Si, Be, Mg, He, Ne, Ar Draw the electronic configuration of the first 20 elements, using both diagrams and numbers.

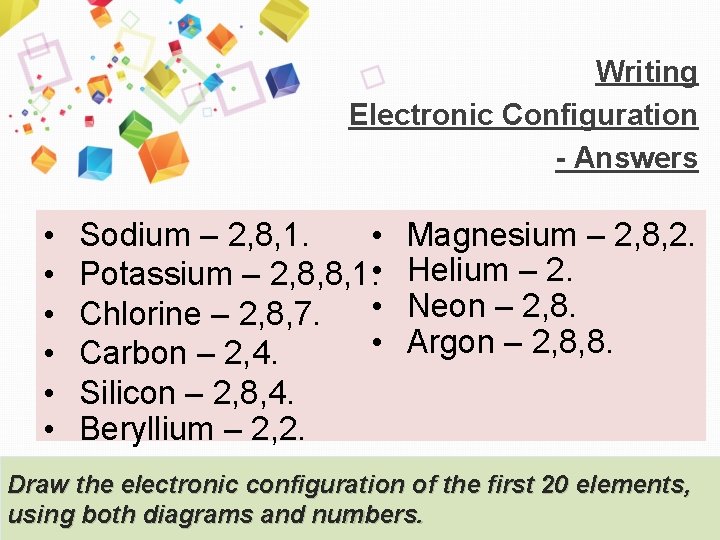
Writing Electronic Configuration - Answers • • • Sodium – 2, 8, 1. • Potassium – 2, 8, 8, 1. • • Chlorine – 2, 8, 7. • Carbon – 2, 4. Silicon – 2, 8, 4. Beryllium – 2, 2. Magnesium – 2, 8, 2. Helium – 2. Neon – 2, 8. Argon – 2, 8, 8. Draw the electronic configuration of the first 20 elements, using both diagrams and numbers.

Writing Electronic Configuration - Answers • Can you spot the pattern between the electronic configuration and the group number? • What do you notice about the electronic configuration for the last 3 elements? Answer questions to describe the link between electron configuration and chemical reactivity.

Answer questions to describe the link between electron configuration and chemical reactivity.

A Full Outer Shell • Helium, neon and argon are part of a group of gases known as the noble or inert gases, i. e. they are very unreactive. • The reason why they are very unreactive is because they have a full outer shell of electrons, which makes them very stable. Answer questions to describe the link between electron configuration and chemical reactivity.

Answer questions to describe the link between electron configuration and chemical reactivity.

The Key to Chemistry! Elements react… …to get a full outer shell! Answer questions to describe the link between electron configuration and chemical reactivity.

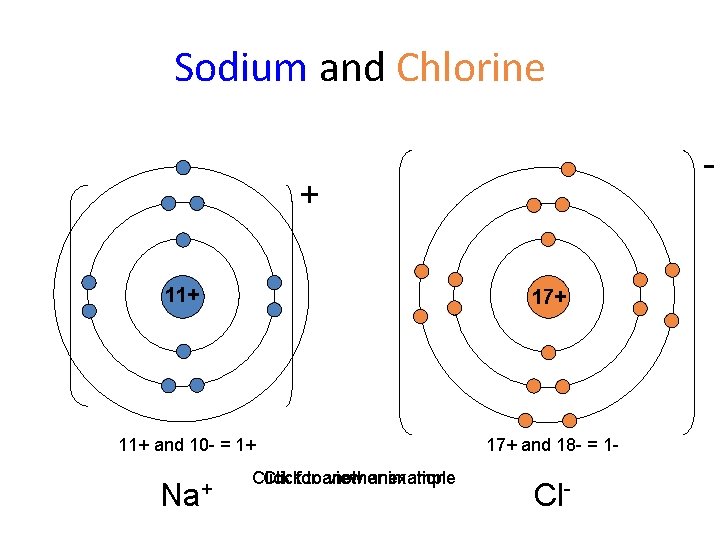
Sodium and Chlorine - + 11+ 17+ 11+ and 10 - = 1+ 17+ and 18 - = 1 - Na+ Clickfor toanother view animation example Cl-

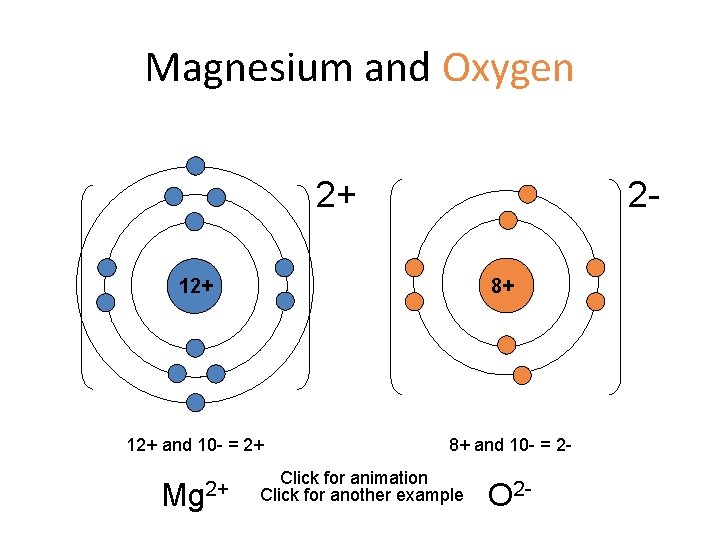
Magnesium and Oxygen 2+ 2 - 12+ 8+ 12+ and 10 - = 2+ Mg 2+ 8+ and 10 - = 2 - Click for animation Click for another example O 2 -

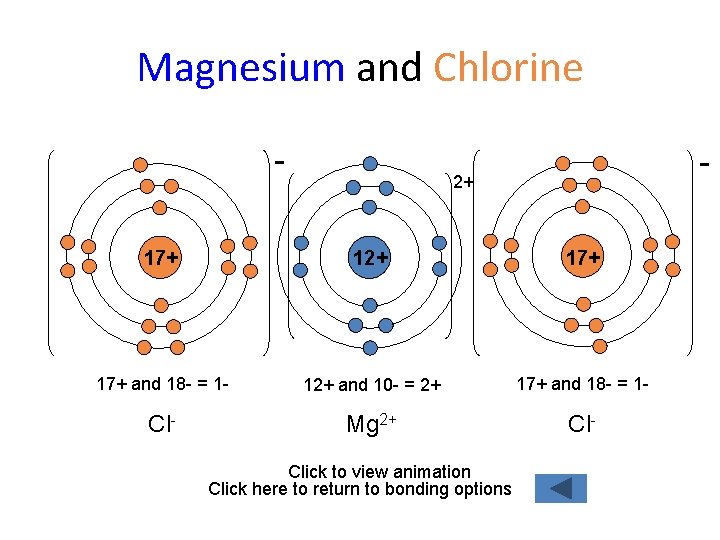
Magnesium and Chlorine - - 2+ 17+ 17+ and 18 - = 1 - 12+ and 10 - = 2+ 17+ and 18 - = 1 - Cl- Mg 2+ Cl- Click to view animation Click here to return to bonding options

HOMEWORK: DUE: Thursday (12. 02. 16) TASKS: 1. Complete any work not finished today. 2. Complete worksheet, “Isotopes. ” HOMEWORK