Electronic Spectroscopy and Vibrationally Mediated Photodissociation of Co
Electronic Spectroscopy and Vibrationally Mediated + Photodissociation of Co (H 2 O), + + Co (D 2 O) and Co (HOD) Systems Abdulkadir Kocak Geoff Austein-Miller Prof. Ricardo B. Metz Chemistry Department
Why Study Co+(H 2 O)? § Strong non-covalent interaction • Co+: 3 d 84 s 0 ground state; 3 d 74 s 1 excited state • Bond strength: 1. 67± 0. 07 e. V* § How does binding to Co+ affect the H 2 O geometry and bonding? • CCSD(T): r. O-H increases by 0. 005 A; θ(H-O-H): increases by 3. 3° • How does this depend on the electronic configuration of metal? § Geometry of the complex • HF planar (C 2 v structure) • B 3 LYP/6 -311+G(3 df, p) ~8 degrees off planar (off from C 2 v) • CCSD(T)/aug-cc-p. VTZ ~10 degrees off planar (off from C 2 v) * N. F. Dalleska, Kenji Honma, L. S. Sunderlin, P. B. Armentrout , JACS 1994 116 (8), 3519 -3528 Chemistry Department 2
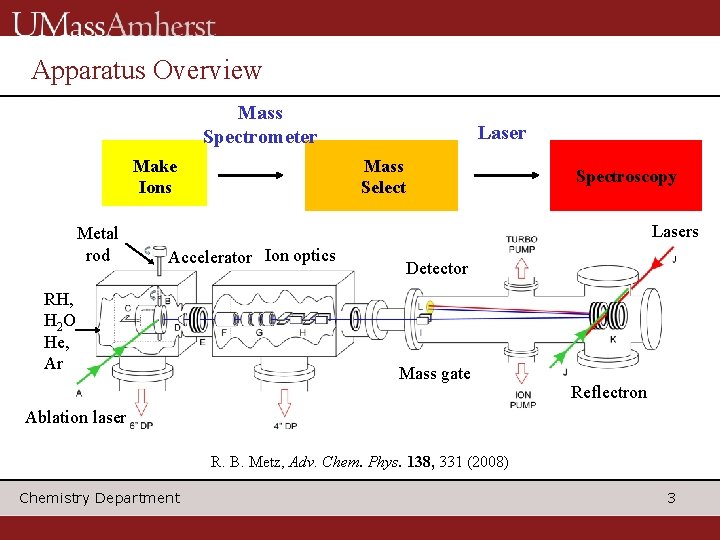
Apparatus Overview Mass Spectrometer Make Ions Metal rod Laser Mass Select Spectroscopy Lasers Accelerator Ion optics RH, H 2 O He, Ar Detector Mass gate Reflectron Ablation laser R. B. Metz, Adv. Chem. Phys. 138, 331 (2008) Chemistry Department 3

Experimental Results Electronic Spectroscopy of + + Co (H 2 O), Co (HOD) and + Co (D 2 O) Complexes Chemistry Department
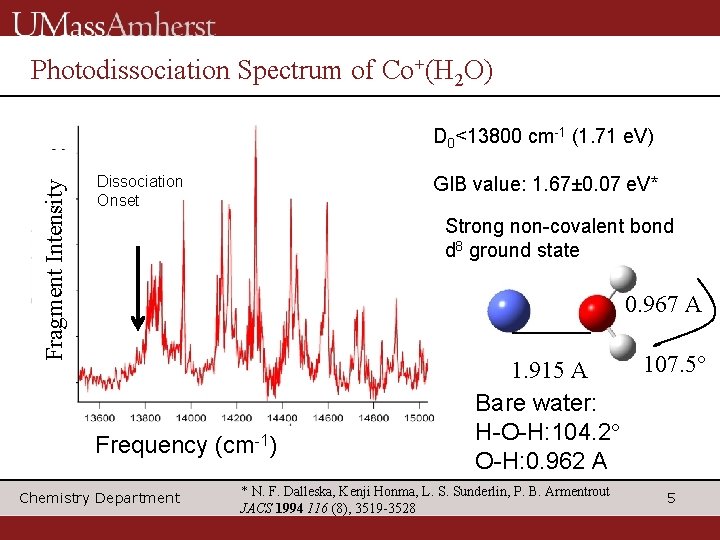
Photodissociation Spectrum of Co+(H 2 O) Fragment Intensity D 0<13800 cm-1 (1. 71 e. V) Dissociation Onset GIB value: 1. 67± 0. 07 e. V* Strong non-covalent bond d 8 ground state 0. 967 A Frequency (cm-1) Chemistry Department 107. 5° 1. 915 A Bare water: H-O-H: 104. 2° O-H: 0. 962 A * N. F. Dalleska, Kenji Honma, L. S. Sunderlin, P. B. Armentrout JACS 1994 116 (8), 3519 -3528 5
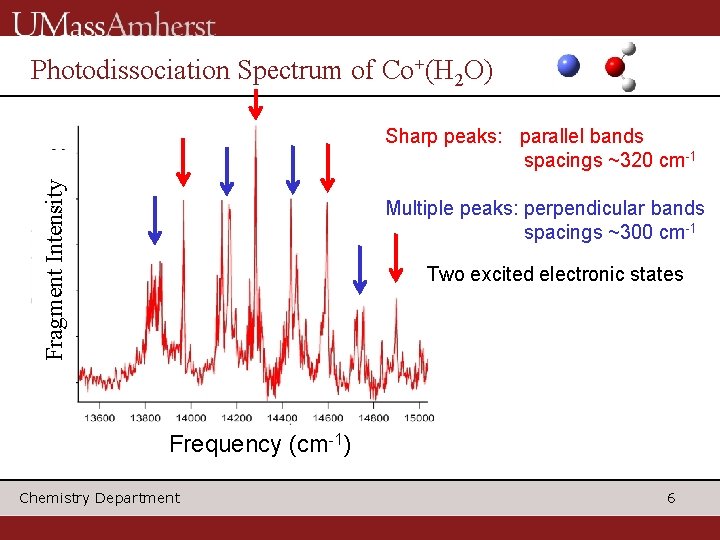
Photodissociation Spectrum of Co+(H 2 O) Fragment Intensity Sharp peaks: parallel bands spacings ~320 cm-1 Multiple peaks: perpendicular bands spacings ~300 cm-1 Two excited electronic states Frequency (cm-1) Chemistry Department 6
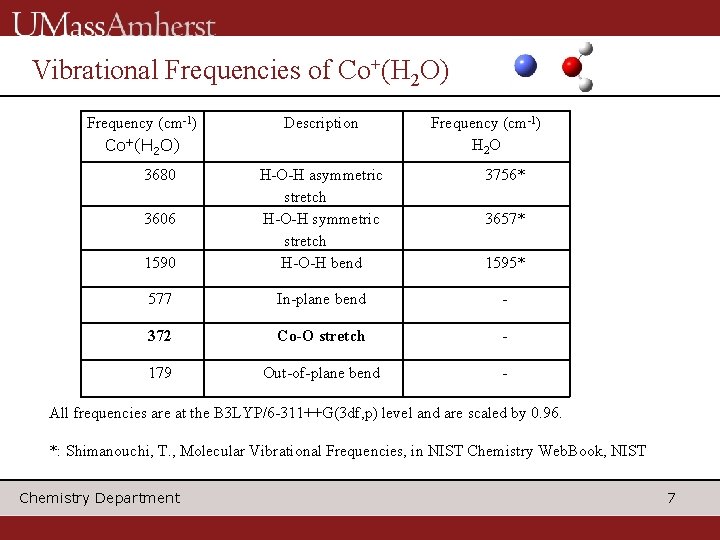
Vibrational Frequencies of Co+(H 2 O) Frequency (cm-1) Co+(H 2 O) 3680 Description Frequency (cm-1) H 2 O 3756* 1590 H-O-H asymmetric stretch H-O-H bend 577 In-plane bend - 372 Co-O stretch - 179 Out-of-plane bend - 3606 3657* 1595* All frequencies are at the B 3 LYP/6 -311++G(3 df, p) level and are scaled by 0. 96. *: Shimanouchi, T. , Molecular Vibrational Frequencies, in NIST Chemistry Web. Book, NIST Chemistry Department 7
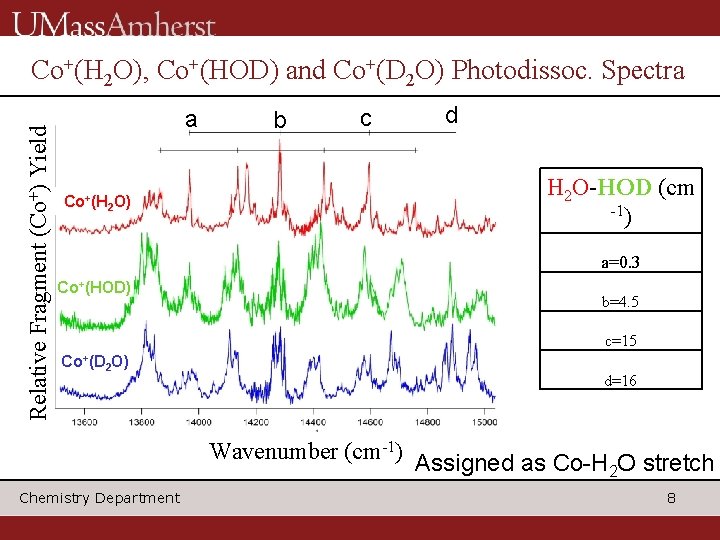
Relative Fragment (Co+) Yield Co+(H 2 O), Co+(HOD) and Co+(D 2 O) Photodissoc. Spectra a b c d H 2 O-HOD (cm -1) Co+(H 2 O) a=0. 3 Co+(HOD) b=4. 5 c=15 Co+(D 2 O) d=16 Wavenumber (cm-1) Chemistry Department Assigned as Co-H 2 O stretch 8
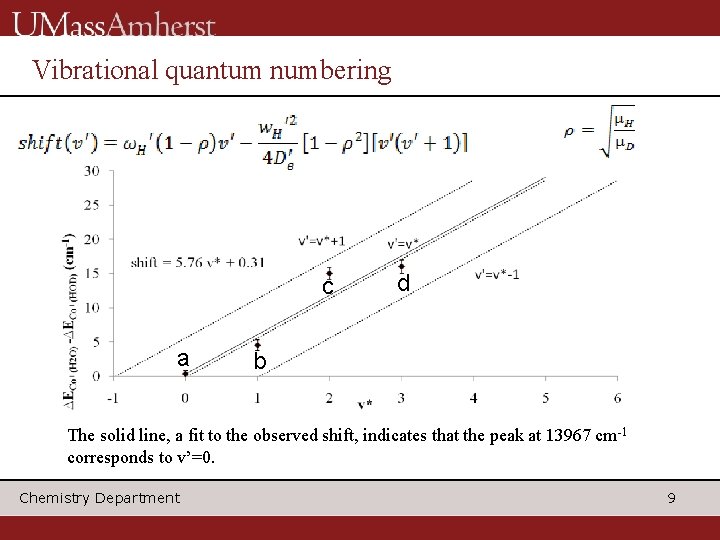
Vibrational quantum numbering c a d b The solid line, a fit to the observed shift, indicates that the peak at 13967 cm-1 corresponds to v’=0. Chemistry Department 9
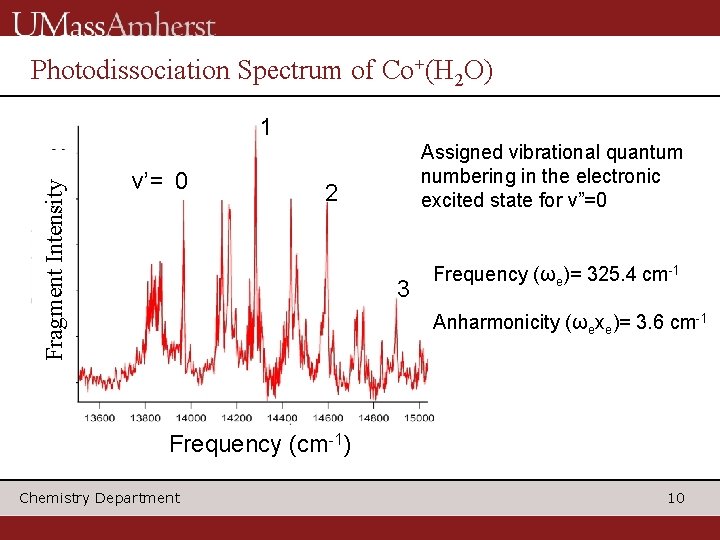
Photodissociation Spectrum of Co+(H 2 O) Fragment Intensity 1 v’= 0 Assigned vibrational quantum numbering in the electronic excited state for v”=0 2 3 Frequency (ωe)= 325. 4 cm-1 Anharmonicity (ωexe)= 3. 6 cm-1 Frequency (cm-1) Chemistry Department 10
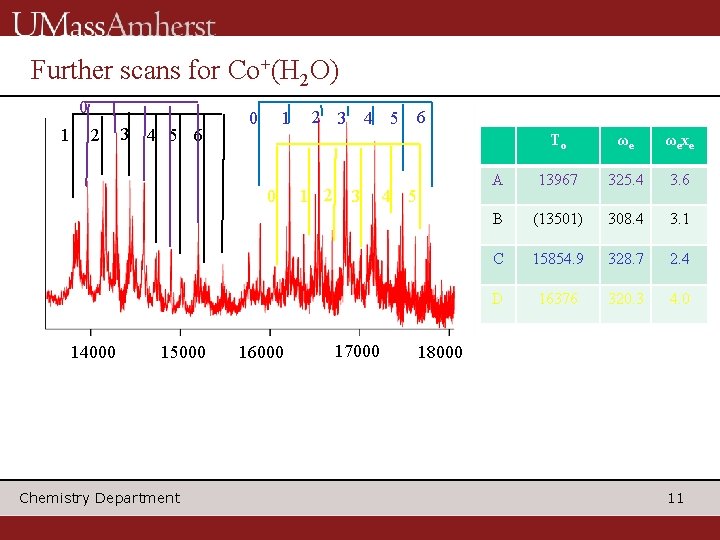
Further scans for Co+(H 2 O) 0 1 2 3 4 5 6 0 14000 15000 Chemistry Department 16000 2 3 4 5 1 2 3 17000 6 4 5 To ωe ωexe A 13967 325. 4 3. 6 B (13501) 308. 4 3. 1 C 15854. 9 328. 7 2. 4 D 16376 320. 3 4. 0 18000 11
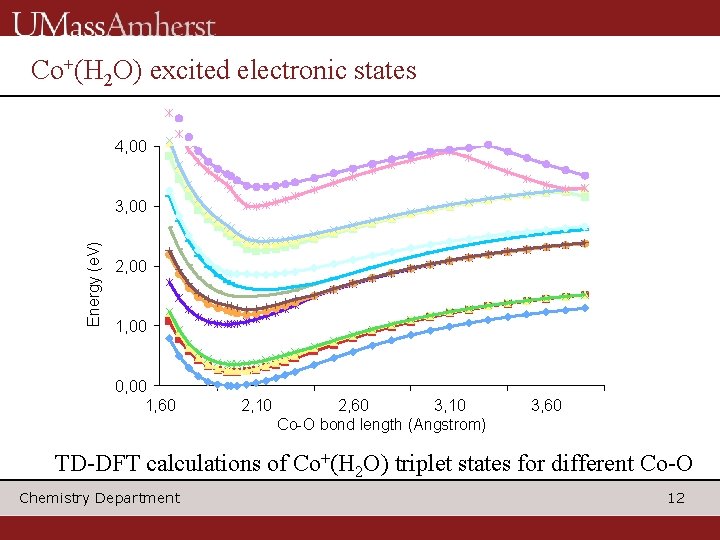
Co+(H 2 O) excited electronic states 4, 00 Energy (e. V) 3, 00 2, 00 1, 00 0, 00 1, 60 2, 10 2, 60 3, 10 Co-O bond length (Angstrom) 3, 60 TD-DFT calculations of Co+(H 2 O) triplet states for different Co-O Chemistry Department 12
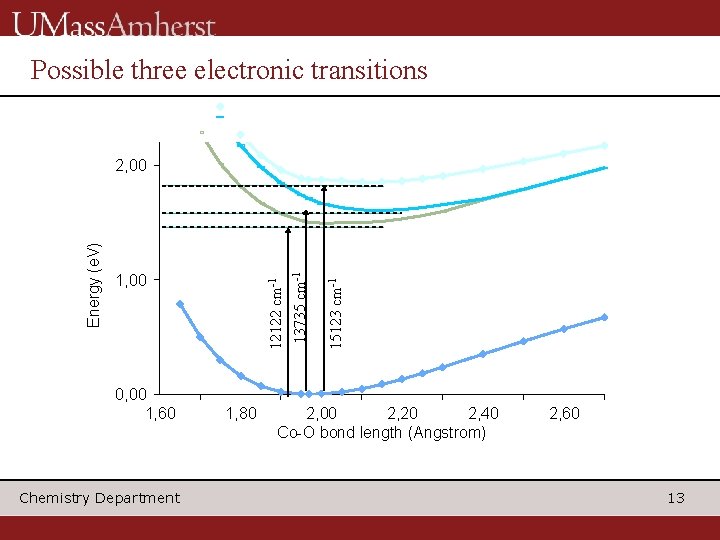
Possible three electronic transitions 0, 00 1, 60 Chemistry Department 1, 80 15123 cm-1 1, 00 12122 cm-1 13735 cm-1 Energy (e. V) 2, 00 2, 20 2, 40 Co-O bond length (Angstrom) 2, 60 13
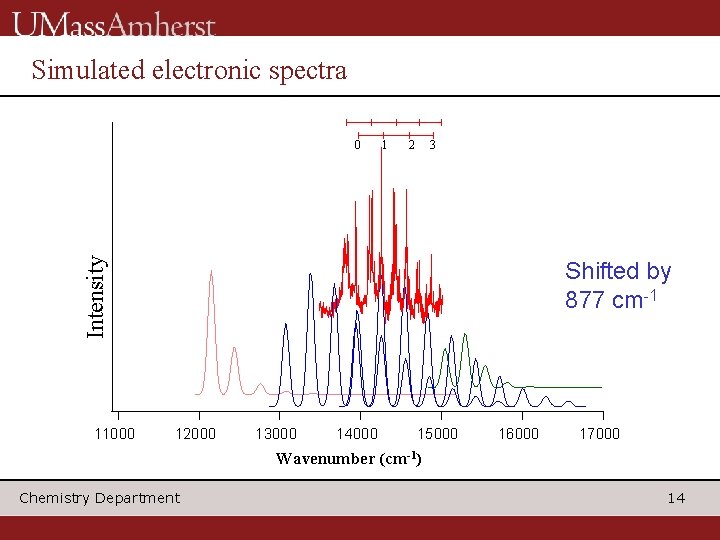
Simulated electronic spectra 1 2 3 Intensity 0 11000 Shifted by 877 cm-1 12000 13000 14000 15000 16000 17000 Wavenumber (cm-1) Chemistry Department 14
Summary And Future Work üDissociation Energy of Co+(H 2 O) determined üFour excited electronic states have been observed üVibrational progression in excited electronic states has been assigned q. We will carry out a rotational analysis Chemistry Department 15

Experimental Results Vibrational Spectroscopy Vibrationally Mediated Photodissociation of + Co (H 2 O) Complexes Chemistry Department
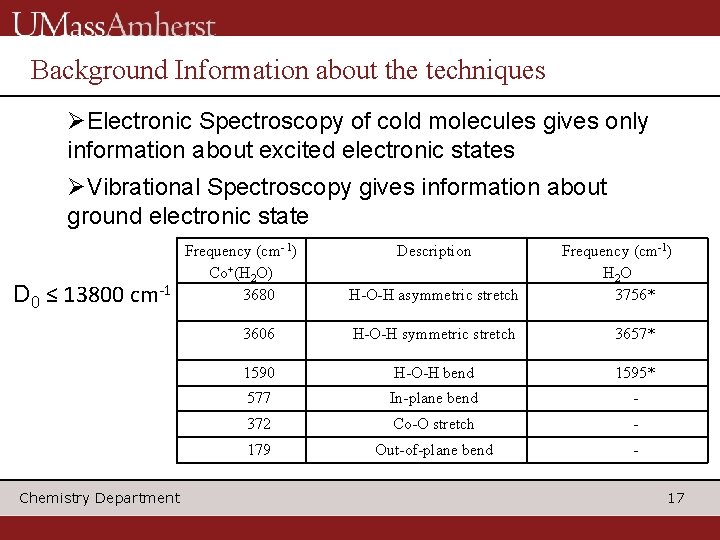
Background Information about the techniques ØElectronic Spectroscopy of cold molecules gives only information about excited electronic states ØVibrational Spectroscopy gives information about ground electronic state D 0 ≤ 13800 Frequency (cm- 1) Co+(H 2 O) 3680 cm-1 Chemistry Department Description H-O-H asymmetric stretch Frequency (cm-1) H 2 O 3756* 3606 H-O-H symmetric stretch 3657* 1590 H-O-H bend 1595* 577 In-plane bend - 372 Co-O stretch - 179 Out-of-plane bend 17
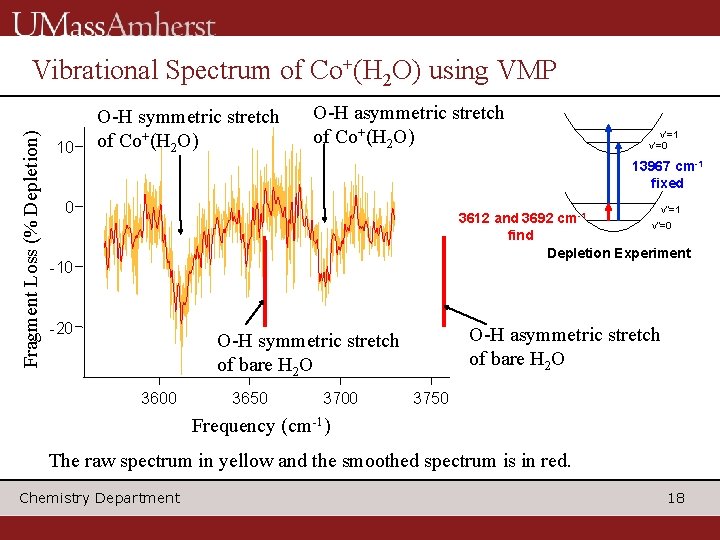
Fragment Loss (% Depletion) Vibrational Spectrum of Co+(H 2 O) using VMP 10 O-H symmetric stretch of Co+(H 2 O) O-H asymmetric stretch of Co+(H 2 O) v’=1 v’=0 13967 cm-1 fixed 0 v”=1 3612 and 3692 cm-1 v”=0 find Depletion Experiment -10 -20 O-H asymmetric stretch of bare H 2 O O-H symmetric stretch of bare H 2 O 3600 3650 3700 3750 Frequency (cm-1) The raw spectrum in yellow and the smoothed spectrum is in red. Chemistry Department 18

Many Thanks To… §Prof. Ricardo B. Metz §Geoff Austein-Miller §Jennifer Silva Daluz §Gokhan Altinay §Wright Pearson §NSF for funding Chemistry Department
- Slides: 19