Electronic Healthcare Record For Clincial Research EHR 4




- Slides: 21

Electronic Healthcare Record For Clincial Research (EHR 4 CR) Semantic interoperability framework WP 4 C. Daniel INSERM UMRS 872 team 20 January 22, 2013 Convergence Meeting: Semantic Interoperability for Clinical Research & Patient Safety in Europe 1

Objective, scope Executive Summary • Objectives – EHR 4 CR platform for reusing EHR data in order to support medical research – Comprehensive business model for governance, acceptance, adoption and sustainability • Duration & budget: 4 years – 7 Mons Euros (+ in kind contribution) • Partners: 33 European academic and industrial partners – 11 Pharmaceutical Companies (members of EFPIA) – 22 Public Partners (Academia, Hospitals and SMEs) January 22, 2013 Convergence Meeting: Semantic Interoperability for Clinical Research & Patient Safety in Europe 2

Partners January 22, 2013 Convergence Meeting: Semantic Interoperability for 3 Clinical Research & Patient Safety in Europe

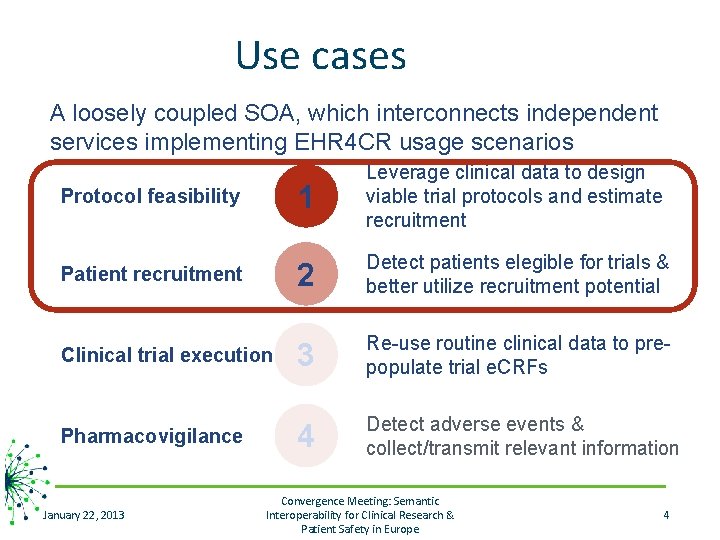
Use cases A loosely coupled SOA, which interconnects independent services implementing EHR 4 CR usage scenarios Protocol feasibility Patient recruitment Clinical trial execution Pharmacovigilance January 22, 2013 1 Leverage clinical data to design viable trial protocols and estimate recruitment 2 Detect patients elegible for trials & better utilize recruitment potential 3 Re-use routine clinical data to prepopulate trial e. CRFs 4 Detect adverse events & collect/transmit relevant information Convergence Meeting: Semantic Interoperability for Clinical Research & Patient Safety in Europe 4

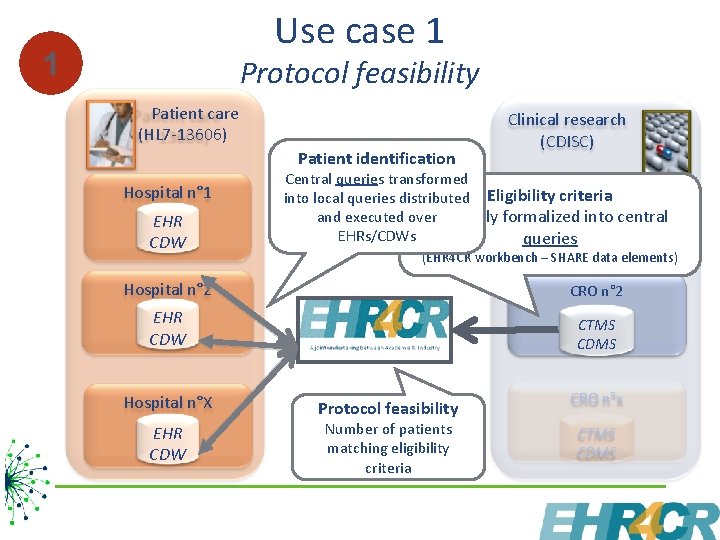
Use case 1 1 Protocol feasibility Patient care (HL 7 -13606) Patient identification Hospital n° 1 EHR CDW Clinical research (CDISC) Central queries transformed into local queries distributed Eligibility criteria CRO n° 1 and executed overmanually formalized into central EHRs/CDWs queries CTMS CDMS (EHR 4 CR workbench – SHARE data elements) Hospital n° 2 CRO n° 2 EHR CDW CTMS CDMS Hospital n°X EHR CDW Protocol feasibility Number of patients matching eligibility criteria CRO n°x CTMS CDMS 5

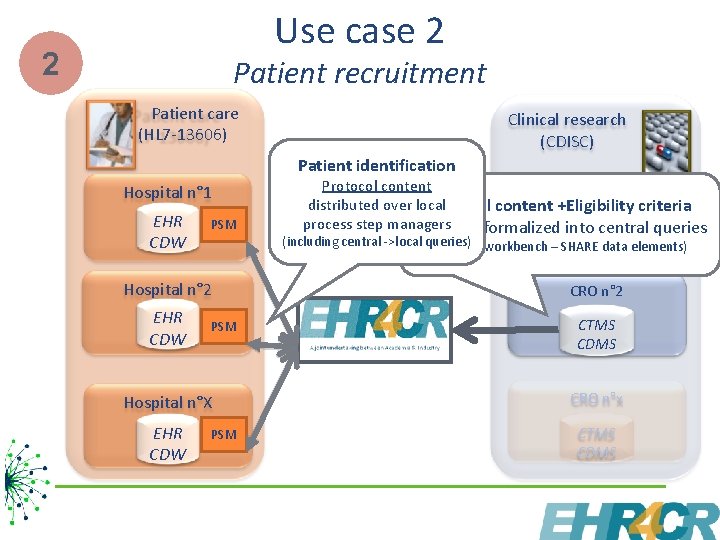
Use case 2 2 Patient recruitment Patient care (HL 7 -13606) Clinical research (CDISC) Patient identification Hospital n° 1 EHR CDW PSM Protocol content CRO n° 1 criteria distributed over local Protocol content +Eligibility process step managers manually formalized into central queries CTMS (including central ->local queries) (EHR 4 CR workbench – SHARE data elements) CDMS Hospital n° 2 CRO n° 2 EHR CDW CTMS CDMS PSM Hospital n°X CRO n°x EHR CDW CTMS CDMS PSM 6

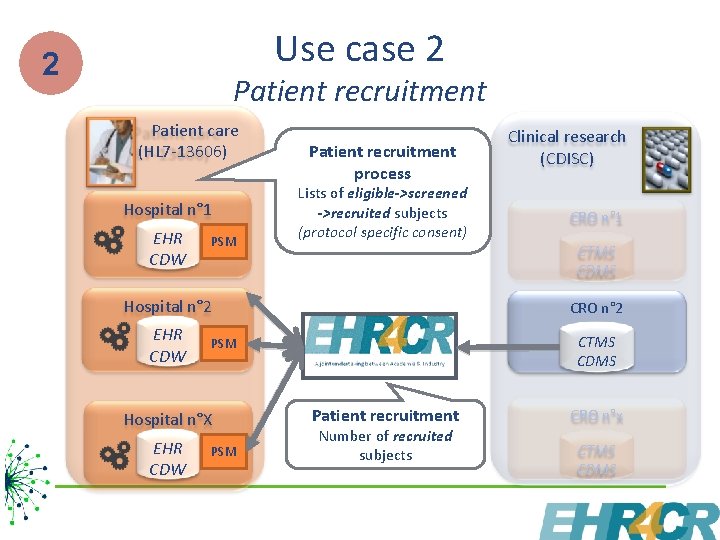
Use case 2 2 Patient recruitment Patient care (HL 7 -13606) Hospital n° 1 EHR CDW PSM Patient recruitment process Lists of eligible->screened ->recruited subjects (protocol specific consent) Clinical research (CDISC) CRO n° 1 CTMS CDMS Hospital n° 2 CRO n° 2 EHR CDW CTMS CDMS PSM Hospital n°X EHR CDW PSM Patient recruitment Number of recruited subjects CRO n°x CTMS CDMS 7

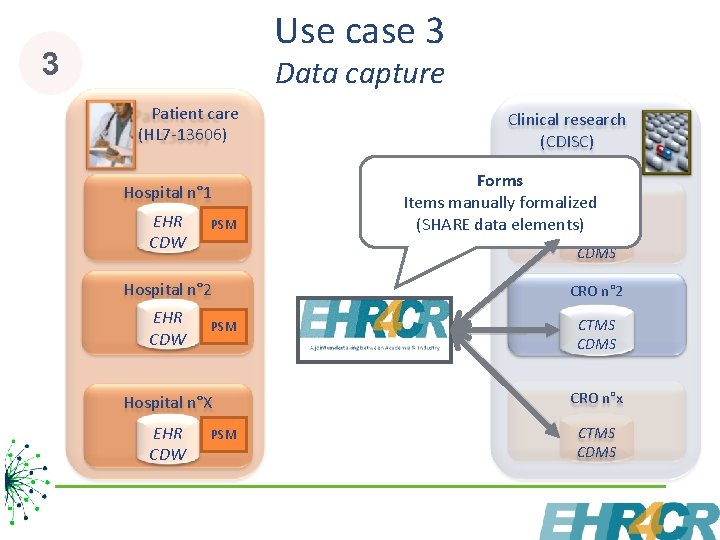
Use case 3 3 Data capture Patient care (HL 7 -13606) Hospital n° 1 EHR CDW PSM Clinical research (CDISC) Forms CRO n° 1 Items manually formalized (SHARE data elements) CTMS CDMS Hospital n° 2 CRO n° 2 EHR CDW CTMS CDMS PSM Hospital n°X CRO n°x EHR CDW CTMS CDMS PSM 8

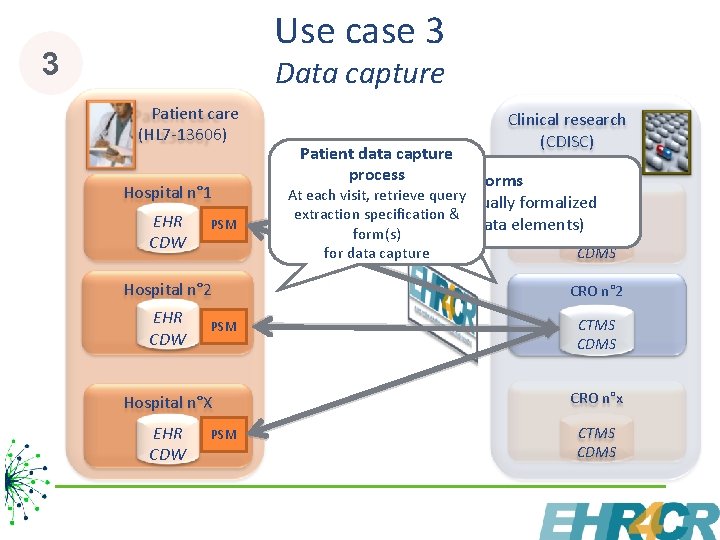
Use case 3 3 Data capture Patient care (HL 7 -13606) Hospital n° 1 EHR CDW PSM Patient data capture process Clinical research (CDISC) Forms At each visit, retrieve query CRO n° 1 Items manually formalized extraction specification & (SHARE data elements) form(s) for data capture CTMS CDMS Hospital n° 2 CRO n° 2 EHR CDW CTMS CDMS PSM Hospital n°X CRO n°x EHR CDW CTMS CDMS PSM 9

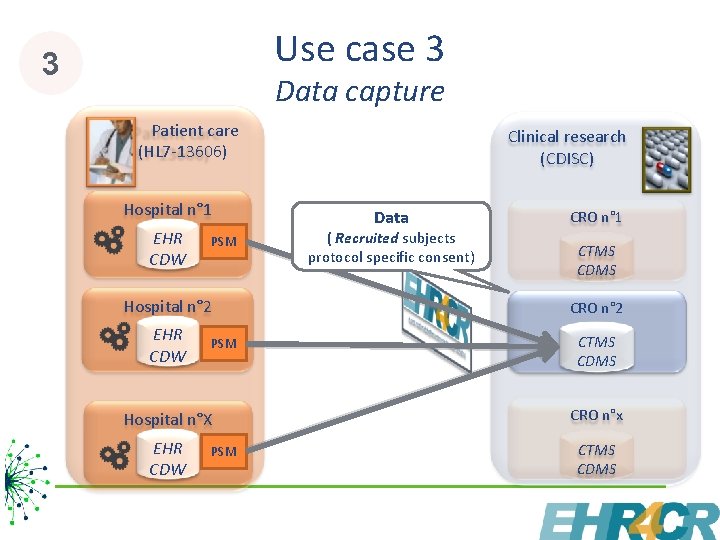
Use case 3 3 Data capture Patient care (HL 7 -13606) Hospital n° 1 EHR CDW PSM Clinical research (CDISC) Data ( Recruited subjects protocol specific consent) CRO n° 1 CTMS CDMS Hospital n° 2 CRO n° 2 EHR CDW CTMS CDMS PSM Hospital n°X CRO n°x EHR CDW CTMS CDMS PSM 10

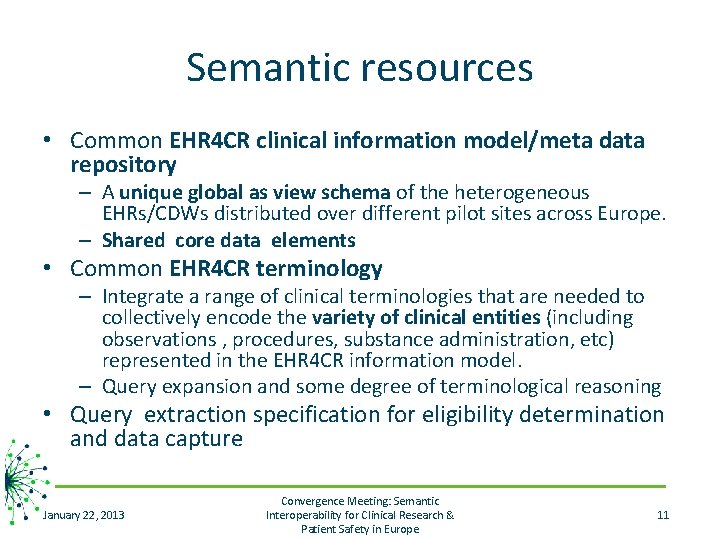
Semantic resources • Common EHR 4 CR clinical information model/meta data repository – A unique global as view schema of the heterogeneous EHRs/CDWs distributed over different pilot sites across Europe. – Shared core data elements • Common EHR 4 CR terminology – Integrate a range of clinical terminologies that are needed to collectively encode the variety of clinical entities (including observations , procedures, substance administration, etc) represented in the EHR 4 CR information model. – Query expansion and some degree of terminological reasoning • Query extraction specification for eligibility determination and data capture January 22, 2013 Convergence Meeting: Semantic Interoperability for Clinical Research & Patient Safety in Europe 11

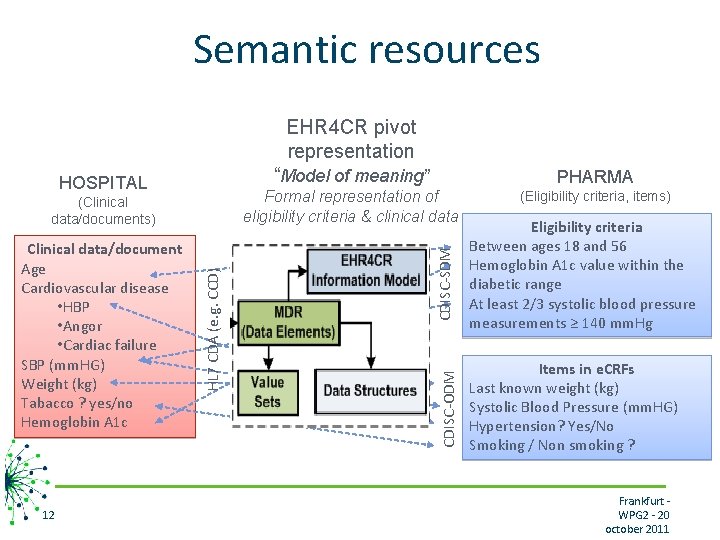
Semantic resources EHR 4 CR pivot representation 12 PHARMA Formal representation of eligibility criteria & clinical data (Eligibility criteria, items) CDISC-SDM Clinical data/document Age Cardiovascular disease • HBP • Angor • Cardiac failure SBP (mm. HG) Weight (kg) Tabacco ? yes/no Hemoglobin A 1 c HL 7 CDA (e. g. CCD) (Clinical data/documents) “Model of meaning” CDISC-ODM HOSPITAL Eligibility criteria Between ages 18 and 56 Hemoglobin A 1 c value within the diabetic range At least 2/3 systolic blood pressure measurements ≥ 140 mm. Hg Items in e. CRFs Last known weight (kg) Systolic Blood Pressure (mm. HG) Hypertension? Yes/No Smoking / Non smoking ? Frankfurt WPG 2 - 20 october 2011

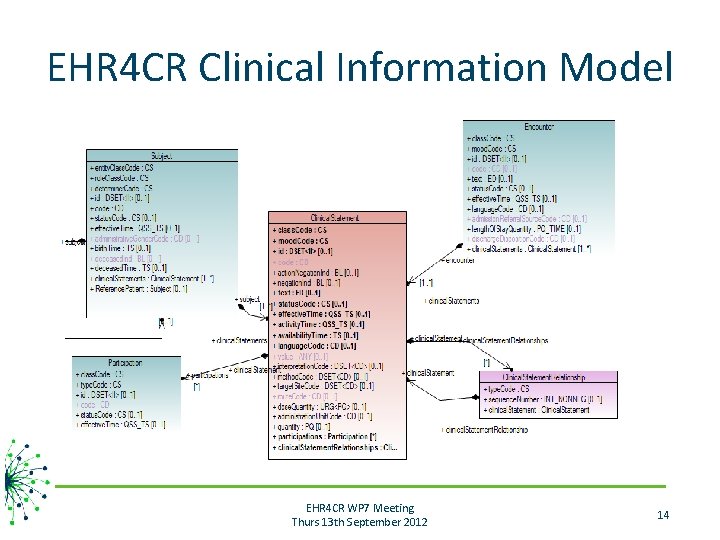
EHR 4 CR Clinical Information Model • Material : “source” models – BRIDG model & HL 7 v 3 models (HL 7 RCRIM WG) • «Study. Design» and «A_Supporting. Clinical. Statement. Universal» models – I 2 b 2 model • Method: Model-driven engineering – Transforming HL 7 v 3 models in UML models and adapt these models to the purpose and scope of the EHR 4 CR project • Multidimensional EHR 4 CR Information Model (ISO 21090 datatypes) • Standardized data elements (pre-processed eligibility criteria of 10 clinical trials) • Tooling: Open Medical Development Framework (OMDF) [Ouagne 10] EHR 4 CR WP 7 Meeting Thurs 13 th September 2012 13

EHR 4 CR Clinical Information Model EHR 4 CR WP 7 Meeting Thurs 13 th September 2012 14

EHR 4 CR meta data repository & terminology (top down) • Material – Reference document templates (e. g. CCD, CDA templates) – Reference terminologies – Patient care: clinical findings, test results, labs, or medications, etc. – Clinical research (Med. DRA, CDASH/ Ontology of Clinical Research) • Method & Tooling – We used Bioportal to upload terminologies – from UMLS (SNOMED CT, LOINC, ICD-10 codes, etc. ) – from other sources (e. g for ATC or Path. Lex) – We developed a data element/value set editor to build a core data element repository EHR 4 CR WP 7 Meeting Thurs 13 th September 2012 15

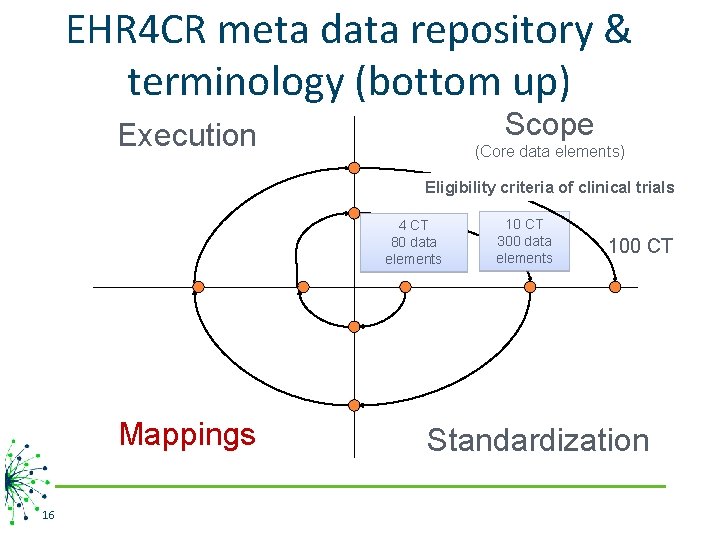
EHR 4 CR meta data repository & terminology (bottom up) Scope Execution (Core data elements) Eligibility criteria of clinical trials 4 CT 80 data elements Mappings 16 10 CT 300 data elements 100 CT Standardization

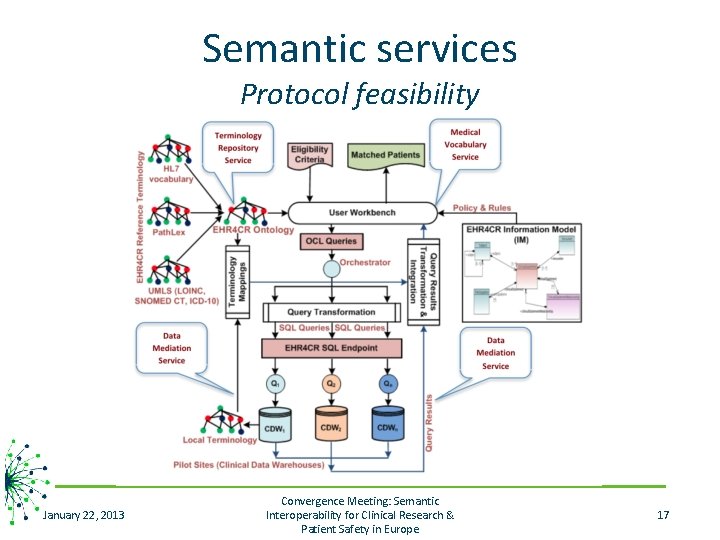
Semantic services Protocol feasibility January 22, 2013 Convergence Meeting: Semantic Interoperability for Clinical Research & Patient Safety in Europe 17

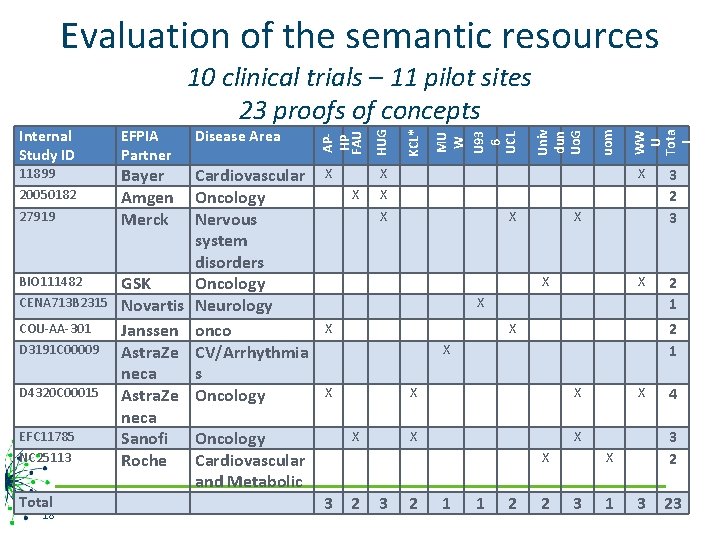
Evaluation of the semantic resources 11899 20050182 27919 BIO 111482 CENA 713 B 2315 COU-AA-301 D 3191 C 00009 D 4320 C 00015 EFC 11785 NC 25113 Total 18 Bayer Amgen Merck Cardiovascular Oncology Nervous system disorders Oncology Neurology onco CV/Arrhythmia s Oncology GSK Novartis Janssen Astra. Ze neca Sanofi Oncology Roche Cardiovascular and Metabolic X X X WW U Tota l uom Univ dun Uo. G MU W U 93 6 UCL KCL* Disease Area HUG EFPIA Partner APHP FAU Internal Study ID 10 clinical trials – 11 pilot sites 23 proofs of concepts X 3 2 3 X 2 1 X X X X X 3 2 1 1 2 2 X 3 1 4 3 23

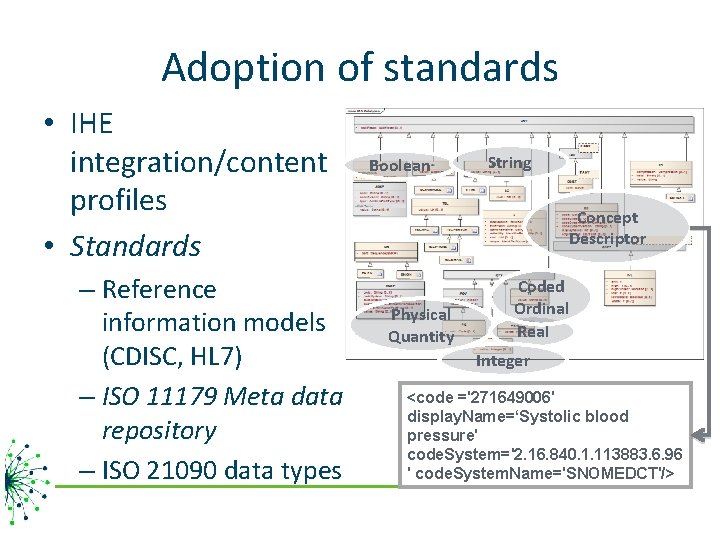
Adoption of standards • IHE integration/content profiles • Standards – Reference information models (CDISC, HL 7) – ISO 11179 Meta data repository – ISO 21090 data types Boolean String Concept Descriptor Physical Quantity Coded Ordinal Real Integer <code ='271649006' display. Name=‘Systolic blood pressure' code. System='2. 16. 840. 1. 113883. 6. 96 ' code. System. Name='SNOMEDCT'/>

Semantic interoperability issues • Building/maintaining semantic resources – Sharing core data elements (CDISC SHARE) – Representing consistently complex clinical information models (clinical data structures templates, data elements) including clinical context • Antibiograms, anatomic pathology cancer checklists (histologic type, grade, TNM, tumor size, etc) – Supporting the mapping process between pivot/local information models & terminologies • Semantic services – Clinical research • Formal representation of eligibility criteria & e. CRF – Mapping medical concepts in eligibility criteria/e. CRFs including highly pre-coordinated terms to standard reference terminologies – Representing temporal constraints – Patient care • Mapping local data structures and/or interface terminologies to pivot models January 22, 2013 Convergence Meeting: Semantic Interoperability for Clinical Research & Patient Safety in Europe 20

Project Information • Project website : http: //www. ehr 4 cr. eu/ • Publications – Ouagne D, Hussain S, Sadou E, Jaulent MC, Daniel C. The Electronic Healthcare Record for Clinical Research (EHR 4 CR) information model and terminology. Stud Health Technol Inform. 2012; 180: 534 -8 – El Fadly A, Rance B, Lucas N, Mead C, Chatellier G, Lastic PY, Jaulent MC, Daniel C. Integrating clinical research with the Healthcare Enterprise: from the RE-USE project to the EHR 4 CR platform. J Biomed Inform. 2011 Dec; 44 Suppl 1: S 94 -102. • Contact information WP 4: Christel. daniel@crc. jussieu. fr January 22, 2013 Convergence Meeting: Semantic Interoperability for Clinical Research & Patient Safety in Europe 21
 Healthcare and the healthcare team chapter 2
Healthcare and the healthcare team chapter 2 Healthcare and the healthcare team chapter 2
Healthcare and the healthcare team chapter 2 Anecdotal record vs running record
Anecdotal record vs running record Next gen emr
Next gen emr Integrated electronic medical record
Integrated electronic medical record How to convert paper br to electronic batch record
How to convert paper br to electronic batch record Electronic health record modernization services
Electronic health record modernization services Scrip exchange
Scrip exchange Electronic field production
Electronic field production Advancing healthcare research
Advancing healthcare research National research university of electronic technology
National research university of electronic technology Microelectronic pill
Microelectronic pill Ppau [email protected]
Ppau [email protected] Sammy ehr podiatry
Sammy ehr podiatry Next gen labs
Next gen labs Rugs to pdpm crosswalk
Rugs to pdpm crosswalk Ihs medical records
Ihs medical records Eszemelyugy
Eszemelyugy Ehr.nisz.hu
Ehr.nisz.hu San francisco hospital
San francisco hospital Gantt chart for ehr implementation
Gantt chart for ehr implementation Ehr nijmegen
Ehr nijmegen