Electronic Cigarettes Hype Versus Science an update Audrey














- Slides: 20

Electronic Cigarettes: Hype Versus Science (an update) Audrey Darville, Ph. D, APRN, CTTS, FAANP Certified Tobacco Treatment Specialist, UK Health. Care Co-Director, Tobacco Prevention & Treatment Division, BREATHE March 2, 2017

Learning Objectives • Describe the current state of electronic smoking products • Identify current use patterns of electronic smoking products • Explore cardiovascular concerns associated with the use of electronic smoking devices

What is an Electronic Smoking Device? Developed in China in 2003; introduced in Europe and the US in 2006 -2007 and banned for sale/distribution in many countries; tested by the FDA in 2009 where inconsistencies were found in labeling versus actual ingredients and the product was refused approval in the U. S.

The Tobacco Control Act gives the FDA jurisdiction to regulate tobacco products (e-cigs, cigars, pipe tobacco, and hookah), and as of August, 2016 they have been deemed tobacco products

Why the Initial Concern? • Cessation treatment claims led FDA to initially pursue regulation as a drug delivery device • FDA testing found nicotine levels varied widely • Toxins (di-ethylene glycol=antifreeze) was found in one sample and ingredients were variable in others • Early reports raised concern for potential harmful effects to the lungs

Over 400 types of Devices • Range from “cig-alike” designs to large tank-style devices with adjustable voltage batteries • Designed & marketed with youth appeal

Heat Not Burn Devices • Initially developed in late 1980’s by tobacco companies; use tobacco not e-juice • No real market appeal until recently • Provide “hit” effect of conventional cigarette that is lacking in electronic cigarettes • Recent market expanding as e-cigarette market is slowing • Industry looking to have them regulated as “reduced-risk” products

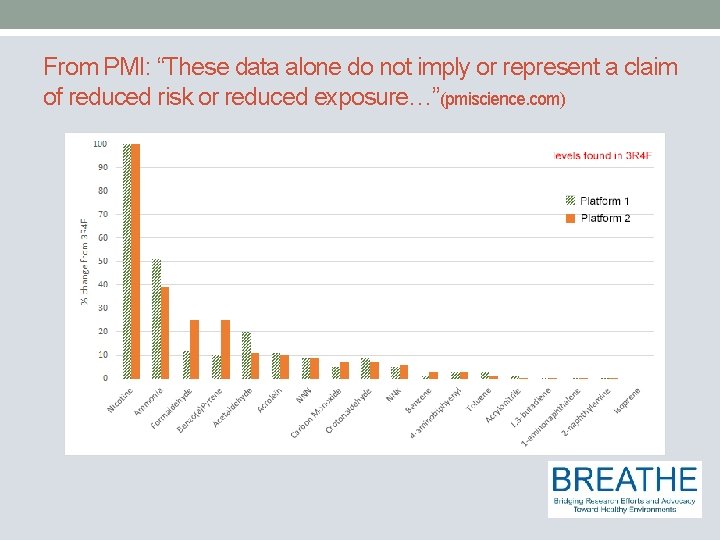
From PMI: “These data alone do not imply or represent a claim of reduced risk or reduced exposure…”(pmiscience. com)

Use Patterns • Primarily users are current or former smokers, many with intent to reduce smoking • Experimentation in non-smokers • No current evidence that long term quit rates are significantly higher than “cold turkey” • Dual/multiple product use is common • Devices are modified for use with substances/drugs other than nicotine • Growing evidence that use may deter cessation

Constituents • Nicotine: Concentrations can vary widely • Overall, significantly fewer chemicals than in combustible cigarettes (40 -60 versus 7000) • Propylene glycol: Principle ingredient in vapor; known lung irritant • Some flavorings associated with known health risks • Formaldehyde: A by-product of heating and oxidation • Aerosolized Particulates: tin, silver, iron, and aluminum. Concentrations of nickel are higher than conventional cigarettes • Oxidizing chemicals

Health Concerns • Lung Effects: Immediate effects on lung function and nitric oxide levels variable but somewhat consistent with conventional cigarettes. Lipoid pneumonia attributed to e-cig use in a young woman • Cardiovascular Concerns: Arrhythmias and hypertension with e-cigarette use have been reported • Cytotoxic/teratogenic Effects: Uncertain at this time but concerns have been raised

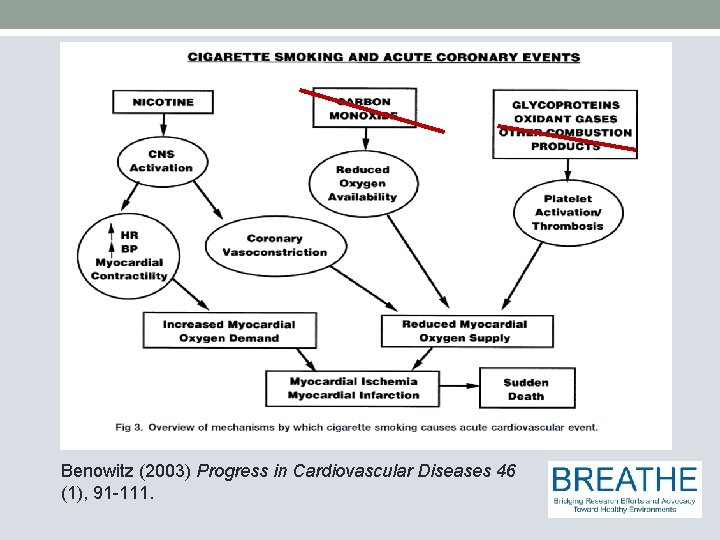
Benowitz (2003) Progress in Cardiovascular Diseases 46 (1), 91 -111.

Cardiovascular Effect “Red Flags” • Oxidative stress markers are elevated with even short term use (Carnevale, et al, 2016, Chest DOI: http: //dx. doi. org/10. 1016/j. chest. 2016. 04. 012) • Chronic use is associated with persistent increases in oxidative stress and sympathetic stimulation in young, healthy subjects (Moheimani, et al, 2017; JAMA Cardiol. doi: 10. 1001/jamacardio. 2016. 5303) • Particulates and carbonyls such as formaldehyde, acetone, acrolein, and butanol in ecigarettes are associated with impaired regulation of blood pressure, increased clotting, and accelerated formation of atherosclerotic lesions (Bhatnagar, 2016, doi: 10. 1007/s 12170 -016 -0505 -6)

The Harm Reduction Debate • Current controversy in tobacco control and public health communities • Perceived as less harmful, but harms are largely untested with controlled studies • Multiple studies are ongoing • Many devices developed/marketed by the tobacco industry • Long range impact on tobacco prevalence & cessation rates is debated and largely unknown

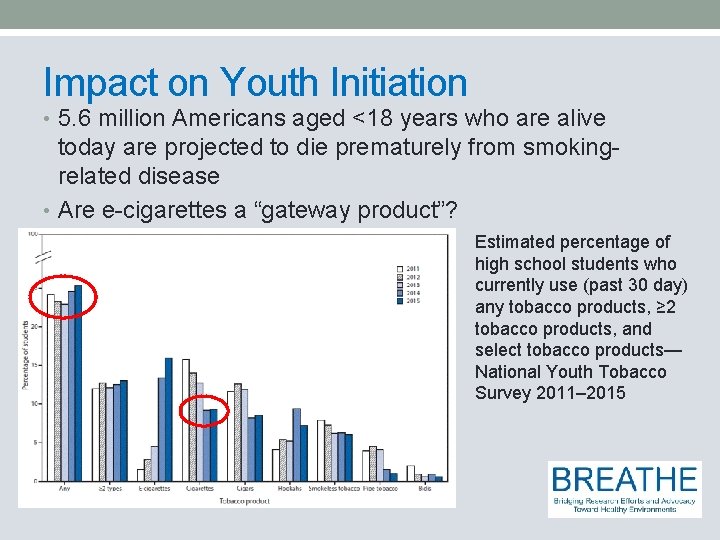
Impact on Youth Initiation • 5. 6 million Americans aged <18 years who are alive today are projected to die prematurely from smokingrelated disease • Are e-cigarettes a “gateway product”? Estimated percentage of high school students who currently use (past 30 day) any tobacco products, ≥ 2 tobacco products, and select tobacco products— National Youth Tobacco Survey 2011– 2015

In Summary • Safety regulations and standards regarding electronic cigarettes and vaping devices are pending: Product use is not “risk free” but “risk unknown” at this time • Nicotine is highly addictive and has been found in e-juices claiming to be nicotine free • There is increasing concern that e-cigarettes are a gateway to other forms of tobacco use in youth • There is no solid evidence that e-cigarettes help people quit smoking and growing evidence that they may be deterring cessation in certain cases • The tobacco industry is investing heavily in “Reduced-Risk Products”

Association for the Treatment of Tobacco Use and Dependence An organization of providers dedicated to the promotion of and increased access to evidence-based tobacco treatment for the tobacco user. www. attud. org

BREATHE Tobacco Treatment Specialist Training Program: An Intensive Evidence-Based Training Program for Health Professionals • Teaches the core competencies for tobacco treatment specialists developed by the Association for the Treatment of Tobacco Use and Dependence • The online course with approximately twenty-five hours of instruction time, which is broken down into five sequential modules • Currently piloting/applying for accreditation but will reopen registration in late spring/early summer • Contact Audrey Darville (audrey. darville@uky. edu) for more information

If interested in clinical case studies see: Clinical Case Conference Electronic cigarettes: a review of safety and clinical issues Michael Weaver, MD, FASAM, Alison Breland, Ph. D, Tory Spindle, BS, & Thomas Eissenberg, Ph. D NIH Public Access Manuscript: J Addict Med. 2014; 8(4): 234– 240. doi: 10. 1097/ADM. 000000043.

Selected References: Bhatnagar A. E-cigarettes and cardiovascular disease risk: evaluation of evidence, policy implications, and recommendations. Curr Cardiovasc Risk Rep. 2016; 10: 1 -10. Benowitz, N. L. , & Goniewicz, M. L. (2013). The Regulatory Challenge of Electronic Cigarettes. JAMA. doi: 10. 1001/jama. 2013. 109501 Bullen, C. , et al. (2013). Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. doi: 10. 1016/S 01406736(13)61842 -5 Carnevale R, Sciarretta S, Violi F, Nocella C, Loffredo L, Perri L, Peruzzi M, Marullo AGM, De Falco E, Chimenti I, Valenti V, Biondi. Zoccai G, Frati G, Acute impact of tobacco versus electronic cigarette smoking on oxidative stress and vascular function, CHEST (2016), doi: 10. 1016/j. chest. 2016. 04. 012. England LJ, et al. (2015) Nicotine and the Developing Human: A Neglected Element in the Electronic Cigarette Debate. Am J Prev Med. 2015; doi 10. 1016/j. amepre. 2015. 015 Goniewicz, M. L. , et al. (2013). Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. doi: 10. 1136/tobaccocontrol-2012 -050859 Grana R, et al. (2014) E-Cigarettes: A Scientific Review. Circulation; 129(19): 1972 -1986. Hajek P, et al. (2014) Electronic cigarettes: review of use, content, safety, effects on smokers and potential for harm and benefit. Addiction; 109(11): 1801 -1810. Mc. Robbie H, et al. (2015). Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst Rev. 2015; 12: CD 010216. Moheimani RS, Bhetraratana M, Yin F, et al. Increased cardiac sympathetic activity and oxidative stres in habitual electronic cigarette users: implications for cardiovascular risk [published online February 1, 2017]. JAMA Cardiol. doi: 10. 1001/jamacardio. 2016. 5303. Vardavas CI, et al. Acute pulmonary effects of using an electronic cigarette: impact on respiratory flow resistance, impedance and exhaled nitric oxide. Chest. 2012; 141(6): 1400 -1406. Williams, M. , et al. (2013). Metal and Silicate Particles Including Nanoparticles Are Present in Electronic Cigarette Cartomizer Fluid and Aerosol. PLo. S One, 8(3), e 57987. doi: 10. 1371/journal. pone. 0057987 Zarwertailo, L. , Pavlov, D. , Ivanova, A. , Ng, G. , Baliunas, D. , Selby, P. , (2017). Concurrent e-cigarette use during tobacco dependence treatment in primary care settings: Association with smoking cessation at 3 - and 6 -months. NTR 19(2): 183 -189. Zhu SH, et al. (2014). Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control; 23(Suppl 3): iii 3 -9.