Electronic and Optoelectronic Polymers WenChang Chen Department of










- Slides: 72

Electronic and Optoelectronic Polymers Wen-Chang Chen Department of Chemical Engineering Institute of Polymer Science and Engineering National Taiwan University

Outlines l History of Conjugated Polymers l Electronic Structures of Conjugated Polymers l Polymer Light-emitting Diodes l Polymer-based Thin Film Transistors l Polymer-based Photovoltaics

What’s Transistor? Transistor A device composed of semiconductor materials that amplifiers a signal or opens or close circuit. l The key ingredient of all digital circuits, including computers. l Today’s microprocessors contains tens of millions of microscopic transistors. l Field-Effect Transistor l A voltage applied between the gate and source controls the current flowing between the source and drain

What’s Transistor? Field effect transistor works like a drain

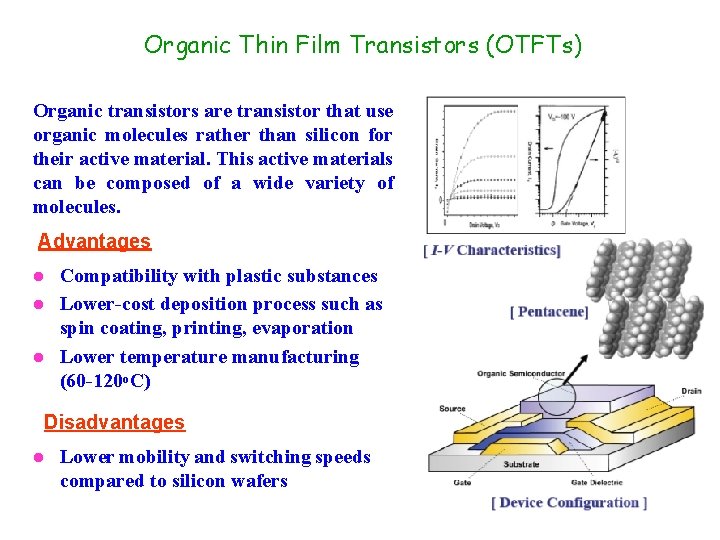
Organic Thin Film Transistors (OTFTs) Organic transistors are transistor that use organic molecules rather than silicon for their active material. This active materials can be composed of a wide variety of molecules. Advantages Compatibility with plastic substances l Lower-cost deposition process such as spin coating, printing, evaporation l Lower temperature manufacturing (60 -120 o. C) l Disadvantages l Lower mobility and switching speeds compared to silicon wafers

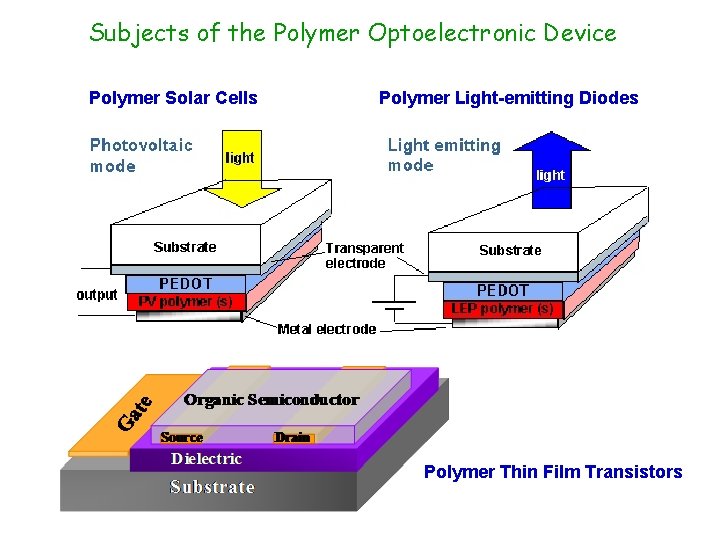
Subjects of the Polymer Optoelectronic Device Polymer Solar Cells Polymer Light-emitting Diodes Polymer Thin Film Transistors

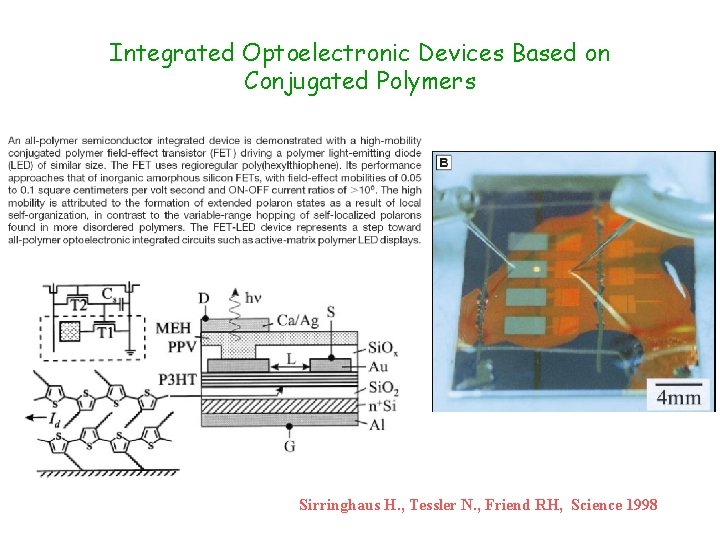
Integrated Optoelectronic Devices Based on Conjugated Polymers Sirringhaus H. , Tessler N. , Friend RH, Science 1998

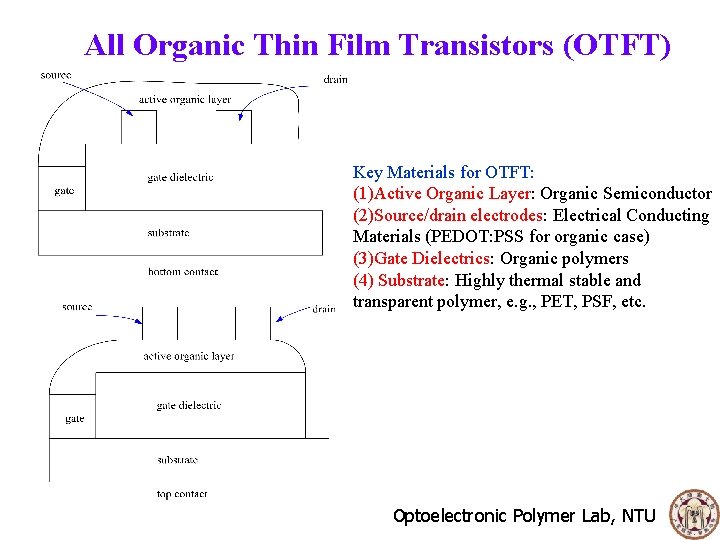
All Organic Thin Film Transistors (OTFT) Key Materials for OTFT: (1)Active Organic Layer: Organic Semiconductor (2)Source/drain electrodes: Electrical Conducting Materials (PEDOT: PSS for organic case) (3)Gate Dielectrics: Organic polymers (4) Substrate: Highly thermal stable and transparent polymer, e. g. , PET, PSF, etc. Optoelectronic Polymer Lab, NTU

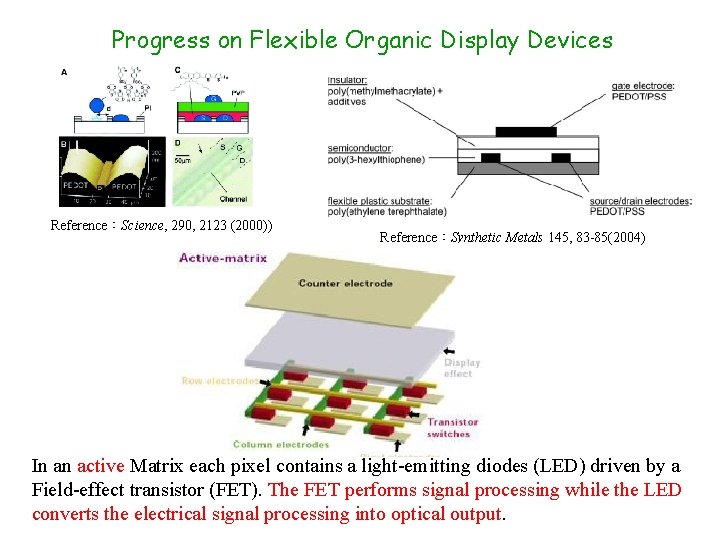
Progress on Flexible Organic Display Devices Reference:Science, 290, 2123 (2000)) Reference:Synthetic Metals 145, 83 -85(2004) In an active Matrix each pixel contains a light-emitting diodes (LED) driven by a Field-effect transistor (FET). The FET performs signal processing while the LED converts the electrical signal processing into optical output.

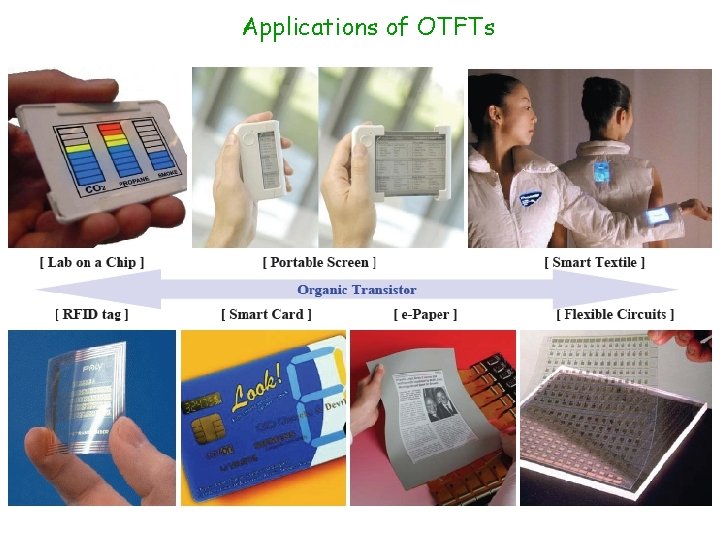
Applications of OTFTs

Applications of OTFTs Flexible TFT arrays enabling technologies for a whole range of applications

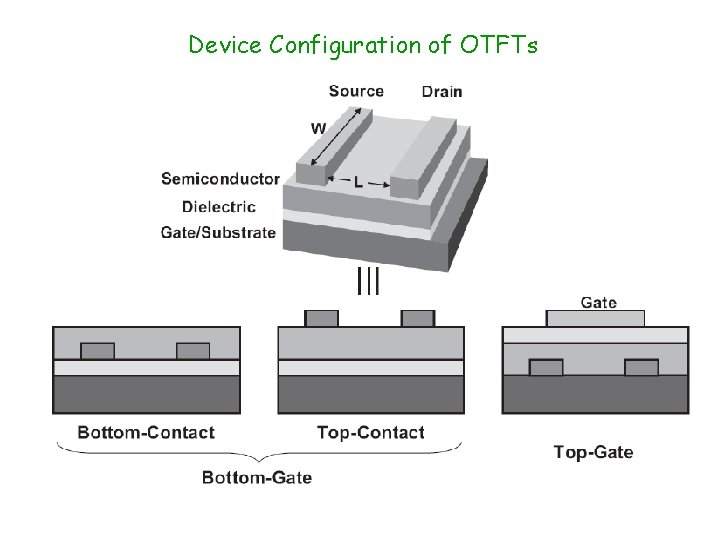
Device Configuration of OTFTs

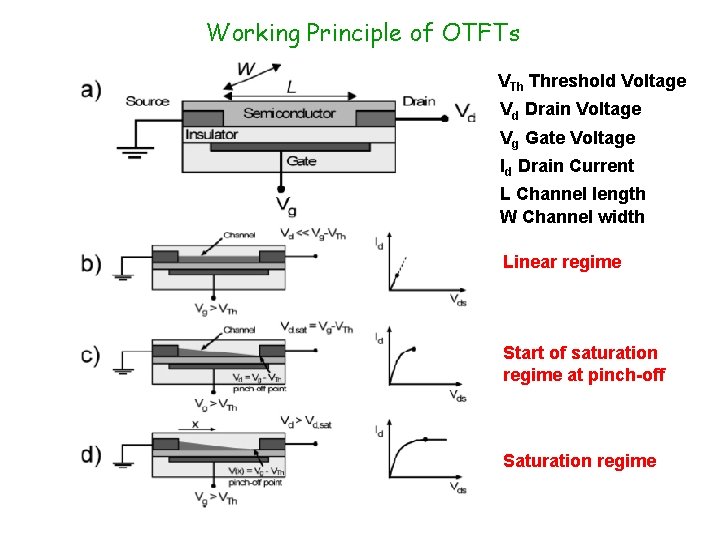
Working Principle of OTFTs VTh Threshold Voltage Vd Drain Voltage Vg Gate Voltage Id Drain Current L Channel length W Channel width Linear regime Start of saturation regime at pinch-off Saturation regime

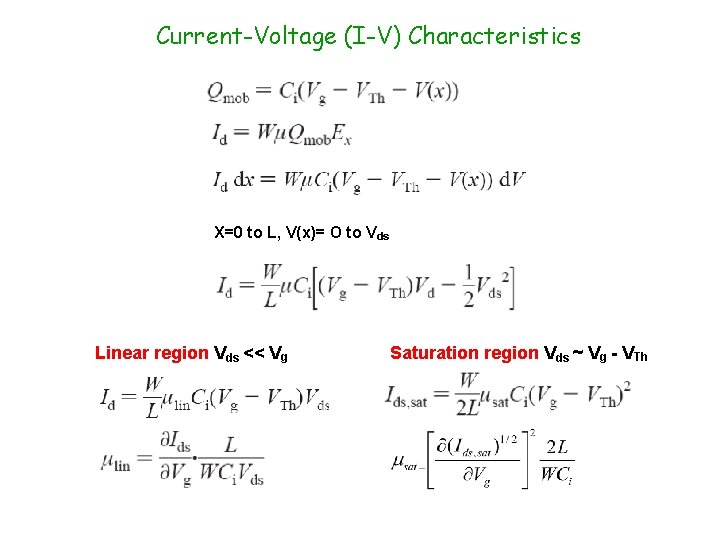
Current-Voltage (I-V) Characteristics X=0 to L, V(x)= O to Vds Linear region Vds << Vg Saturation region Vds ~ Vg - VTh

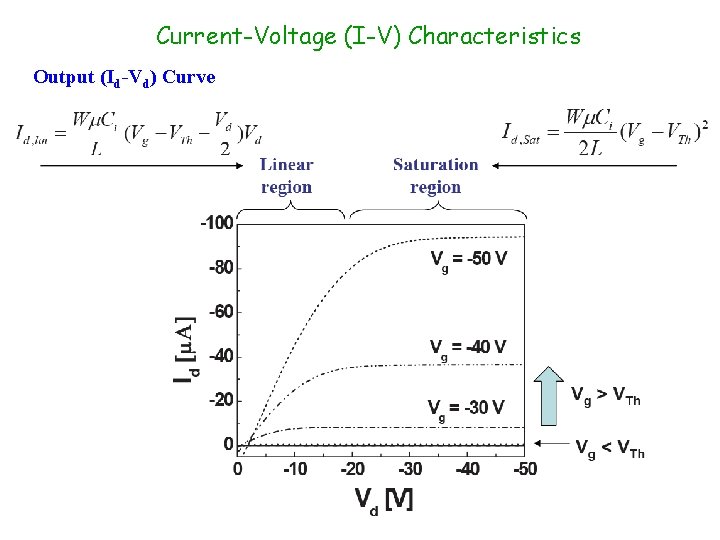
Current-Voltage (I-V) Characteristics Output (Id-Vd) Curve

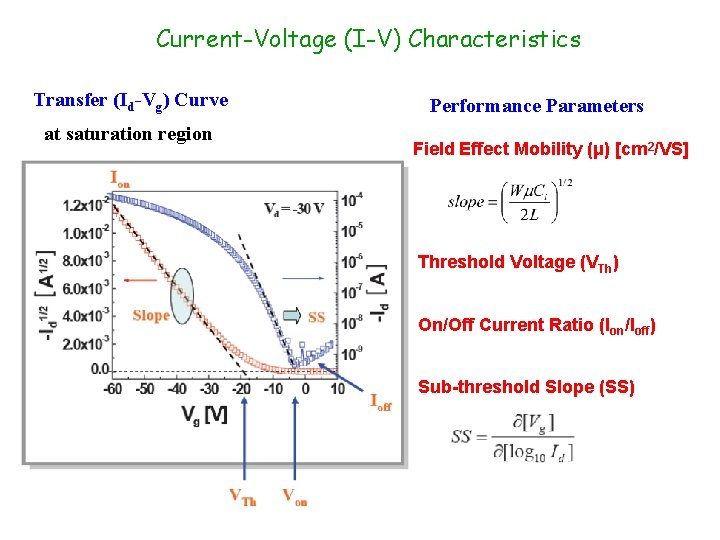
Current-Voltage (I-V) Characteristics Transfer (Id-Vg) Curve at saturation region Performance Parameters Field Effect Mobility (μ) [cm 2/VS] Threshold Voltage (VTh) On/Off Current Ratio (Ion/Ioff) Sub-threshold Slope (SS)

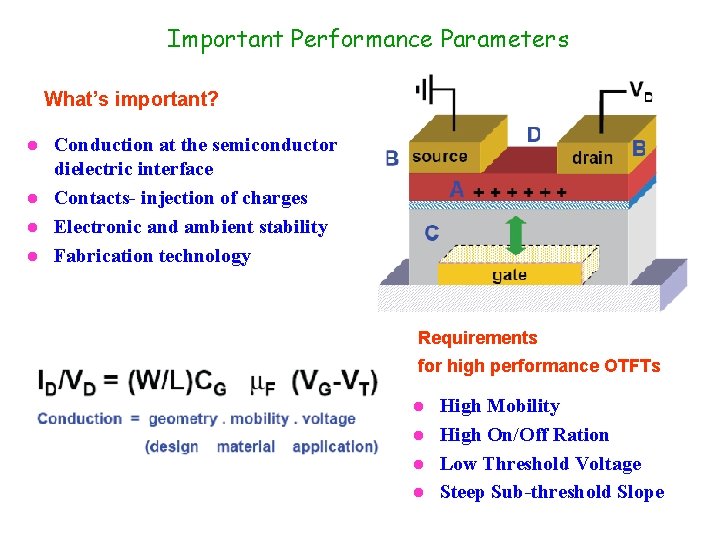
Important Performance Parameters What’s important? Conduction at the semiconductor dielectric interface l Contacts- injection of charges l Electronic and ambient stability l Fabrication technology l Requirements for high performance OTFTs High Mobility l High On/Off Ration l Low Threshold Voltage l Steep Sub-threshold Slope l

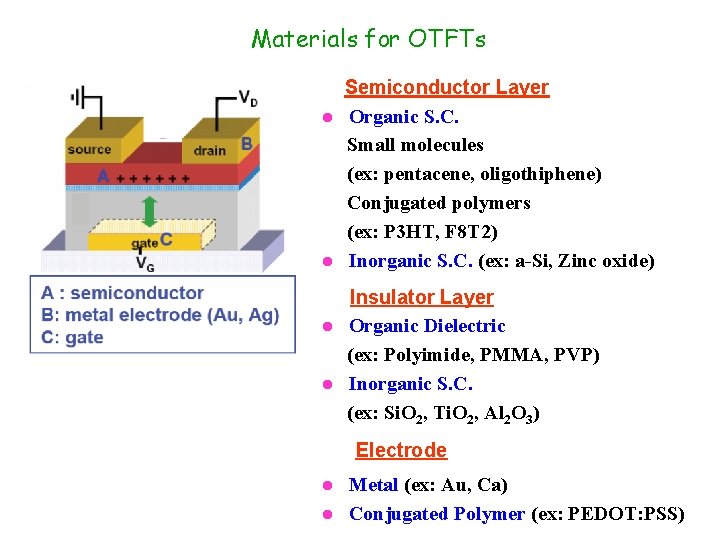
Materials for OTFTs Semiconductor Layer l Organic S. C. Small molecules (ex: pentacene, oligothiphene) Conjugated polymers (ex: P 3 HT, F 8 T 2) l Inorganic S. C. (ex: a-Si, Zinc oxide) Insulator Layer l Organic Dielectric (ex: Polyimide, PMMA, PVP) l Inorganic S. C. (ex: Si. O 2, Ti. O 2, Al 2 O 3) Electrode Metal (ex: Au, Ca) l Conjugated Polymer (ex: PEDOT: PSS) l

Materials Requirements of Organic Semiconductors for OTFT 2 6 l Target: > 1 cm /Vs on/off ratio >10 for n type or p/n type Organic Semiconductors l Conjugated π-Electron System High Electron Affinity ( for n type) or Ambipolar Characteristics (for p/n type) l l Good Intermolecular Electronic Overlap chemical bonding between molecules, molecular symmetry, the symmetry of the crystal packing…. Good Film Forming Properties polycrystalline film be highly oriented so that fast transports direction in the grains lie parallel to the dielectric surface l Chemical Purity charge trapping sites, dopants (increase the conductivity in off state) l Stability device operation (Threshold Voltage Shift), air stability(O 2, H 2 O)

Requirements of Materials for OTFTs

Factors Influencing OTFTs Performance

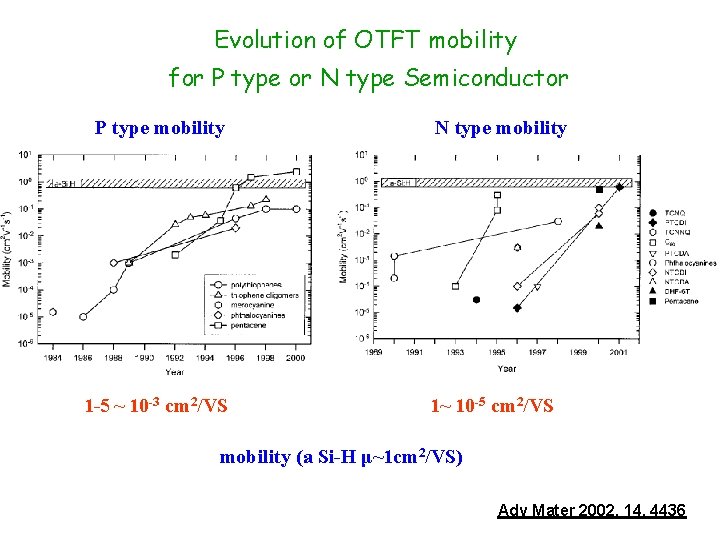
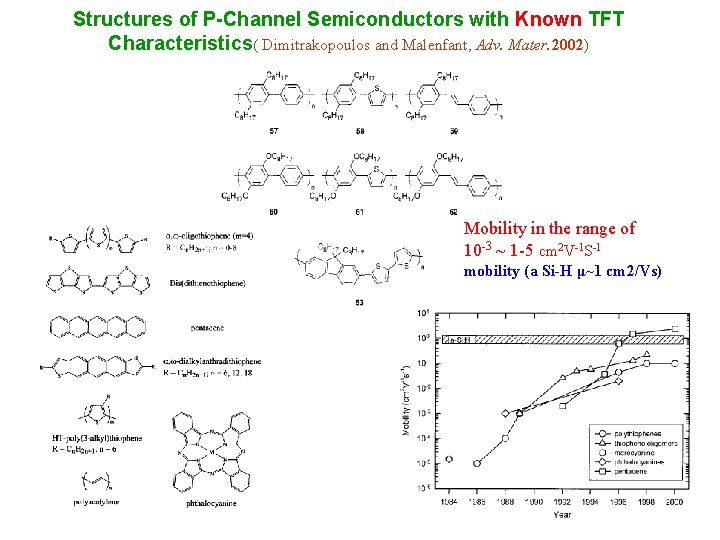
Evolution of OTFT mobility for P type or N type Semiconductor P type mobility 1 -5 ~ 10 -3 cm 2/VS N type mobility 1~ 10 -5 cm 2/VS mobility (a Si-H μ~1 cm 2/VS) Adv Mater 2002, 14, 4436

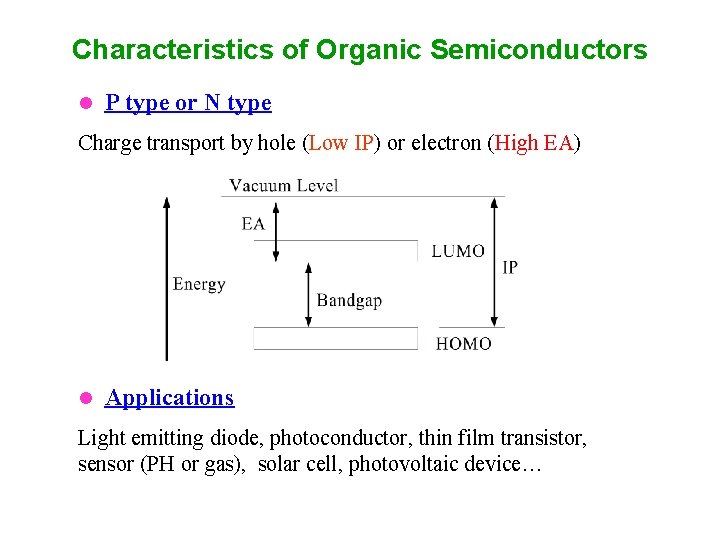
Characteristics of Organic Semiconductors l P type or N type Charge transport by hole (Low IP) or electron (High EA) l Applications Light emitting diode, photoconductor, thin film transistor, sensor (PH or gas), solar cell, photovoltaic device…

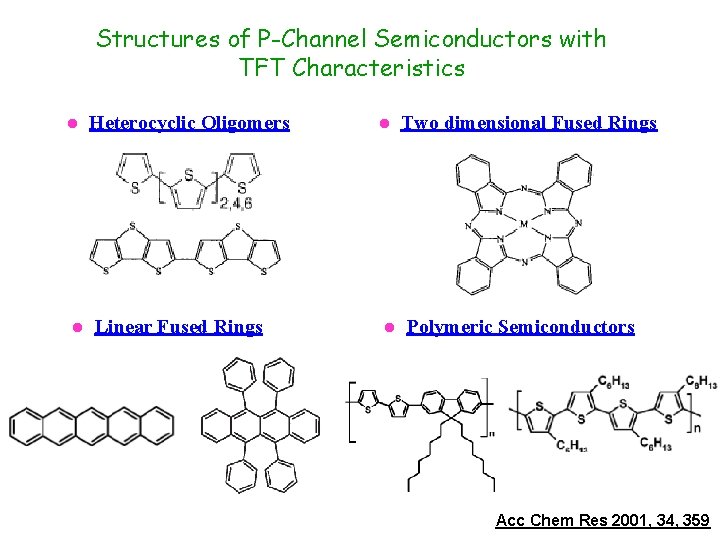
Structures of P-Channel Semiconductors with TFT Characteristics l l Heterocyclic Oligomers Linear Fused Rings l l Two dimensional Fused Rings Polymeric Semiconductors Acc Chem Res 2001, 34, 359

Structures of P-Channel Semiconductors with Known TFT Characteristics( Dimitrakopoulos and Malenfant, Adv. Mater. 2002) Mobility in the range of 10 -3 ~ 1 -5 cm 2 V-1 S-1 mobility (a Si-H μ~1 cm 2/Vs)

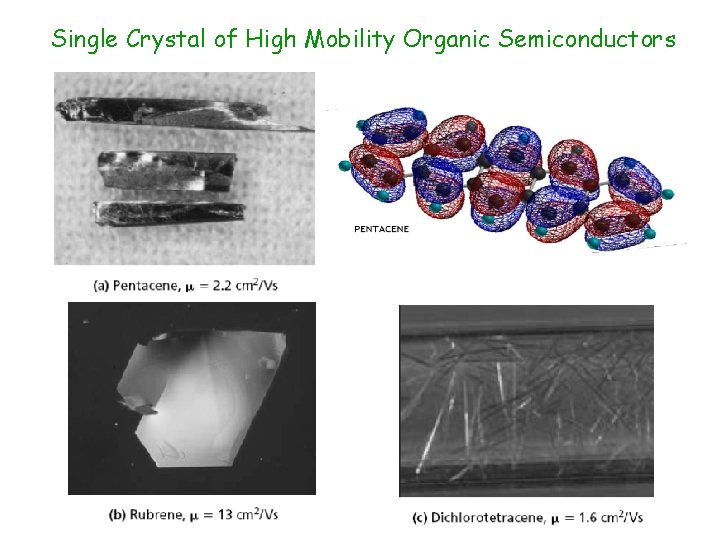
Single Crystal of High Mobility Organic Semiconductors

Materials Requirements for n-Channel Organic Semiconductors l Conjugated π-Electron System with High Electron Affinity (EA > 3. 0 e. V) l Good Intermolecular Electronic Overlap chemical bonding between molecules, molecular symmetry, the symmetry of the crystal packing…. l Good Film Forming Properties polycrystalline film be highly oriented so that fast transports direction in the grains lie parallel to the dielectric surface l Chemical Purity charge trapping sites, dopants l Stability device operation (Threshold Voltage Shift), air stability(O 2, H 2 O) Chem Mater 2004, 16, 4436

Enhancement on the OTFT Characteristics l Materials issues l Materials Design and Preparation(HT%, regioregular, repeating conjugated unit, substituent, synthesis method, refinement) l Key materials Optimization (gate, source, drain, substrate, dielectric) l TFT Structures l Chemical Treatment on dielectric film surface ( silane layer pretreatment, SAMs thiol-based chemical modified contact) l Modifying the TFT structure (bottom contact or top contact) l Processing Optimization l Organic layer deposition (i) vacuum evaporation (ii) spin coating, solution casting, printing l Controlling the deposition parameters (aging, deposition rate, anneal process, solvent quality, channel length, channel dimension, deposition thickness, solvent evaporation temperature)

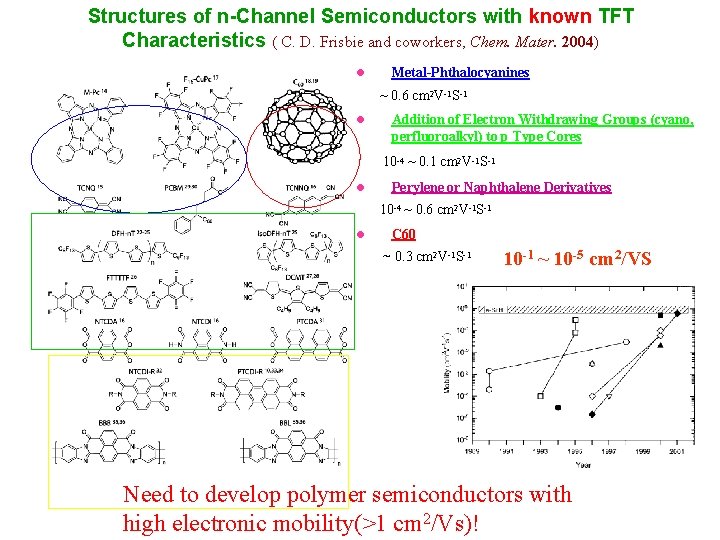
Structures of n-Channel Semiconductors with known TFT Characteristics ( C. D. Frisbie and coworkers, Chem. Mater. 2004) l Metal-Phthalocyanines ~ 0. 6 cm 2 V-1 S-1 l Addition of Electron Withdrawing Groups (cyano, perfluoroalkyl) to p Type Cores 10 -4 ~ 0. 1 cm 2 V-1 S-1 l Perylene or Naphthalene Derivatives 10 -4 ~ 0. 6 cm 2 V-1 S-1 l C 60 ~ 0. 3 cm 2 V-1 S-1 10 -1 ~ 10 -5 cm 2/VS Need to develop polymer semiconductors with high electronic mobility(>1 cm 2/Vs)!

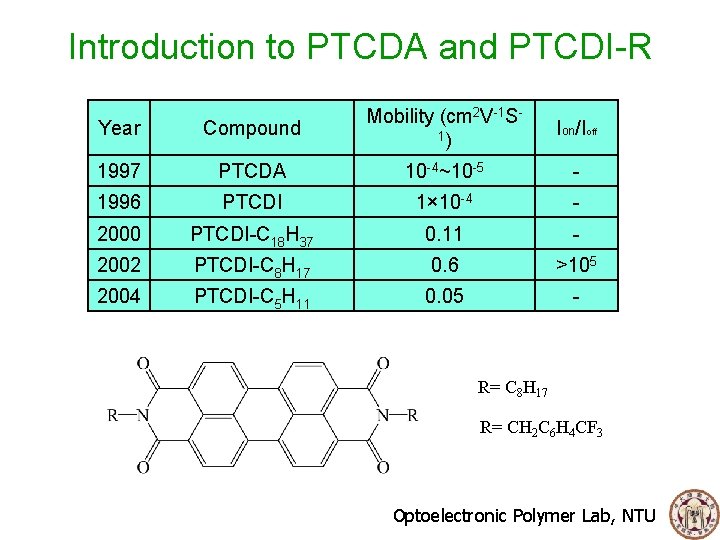
Introduction to PTCDA and PTCDI-R Year Compound Mobility (cm 2 V-1 S 1) 1997 PTCDA 10 -4~10 -5 - 1996 PTCDI 1× 10 -4 - 2000 PTCDI-C 18 H 37 0. 11 - 2002 PTCDI-C 8 H 17 0. 6 >105 2004 PTCDI-C 5 H 11 0. 05 - Ion/Ioff R= C 8 H 17 R= CH 2 C 6 H 4 CF 3 Optoelectronic Polymer Lab, NTU

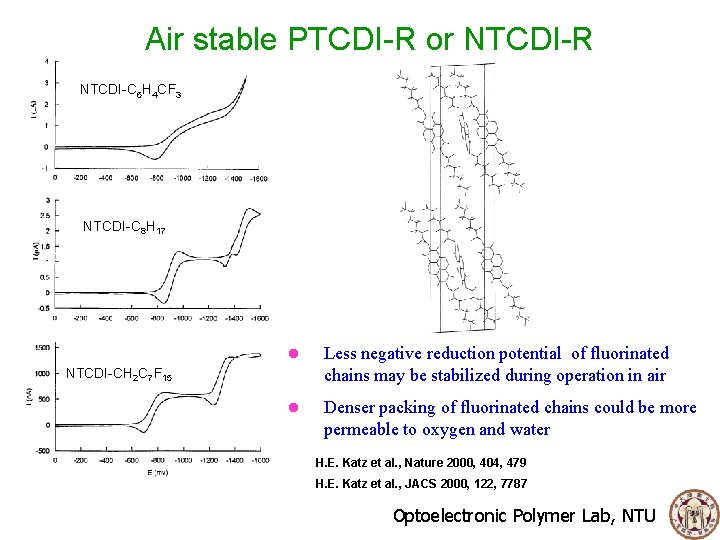
Air stable PTCDI-R or NTCDI-R NTCDI-C 6 H 4 CF 3 NTCDI-C 8 H 17 l Less negative reduction potential of fluorinated chains may be stabilized during operation in air l Denser packing of fluorinated chains could be more permeable to oxygen and water NTCDI-CH 2 C 7 F 15 H. E. Katz et al. , Nature 2000, 404, 479 H. E. Katz et al. , JACS 2000, 122, 7787 Optoelectronic Polymer Lab, NTU

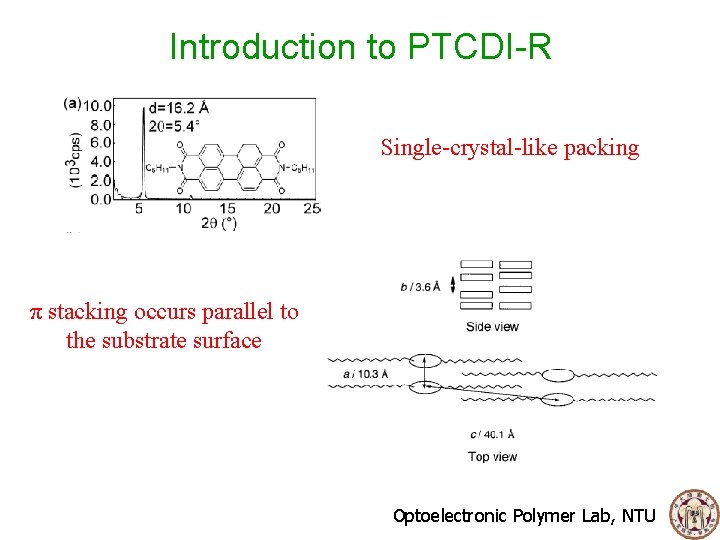
Introduction to PTCDI-R Single-crystal-like packing π stacking occurs parallel to the substrate surface Optoelectronic Polymer Lab, NTU

Why Using PTCDI-R as N Type OTFTs l Single-step synthesis l Impart additional electron withdrawing character to the conjugated backbones to stabilized electron injection. l Provide screening against penetration of environmental contaminants (H 2 O, O 2. . )into the channel region. l The side group could induce a more favorable packing geometry that increases intermolecular overlaps or reduces phonon scattering. Optoelectronic Polymer Lab, NTU

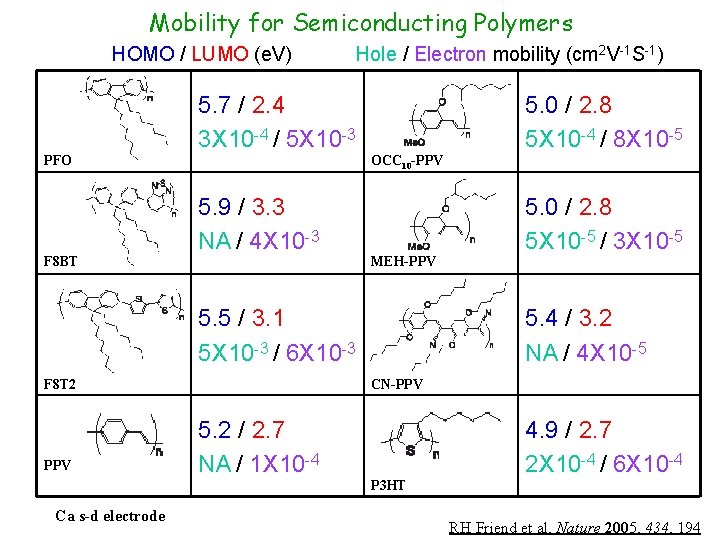
Mobility for Semiconducting Polymers HOMO / LUMO (e. V) Hole / Electron mobility (cm 2 V-1 S-1) 5. 7 / 2. 4 3 X 10 -4 / 5 X 10 -3 PFO F 8 BT 5. 0 / 2. 8 5 X 10 -4 / 8 X 10 -5 OCC 10 -PPV 5. 9 / 3. 3 NA / 4 X 10 -3 MEH-PPV 5. 5 / 3. 1 5 X 10 -3 / 6 X 10 -3 F 8 T 2 PPV 5. 0 / 2. 8 5 X 10 -5 / 3 X 10 -5 5. 4 / 3. 2 NA / 4 X 10 -5 CN-PPV 5. 2 / 2. 7 NA / 1 X 10 -4 4. 9 / 2. 7 2 X 10 -4 / 6 X 10 -4 P 3 HT Ca s-d electrode RH Friend et al, Nature 2005, 434, 194

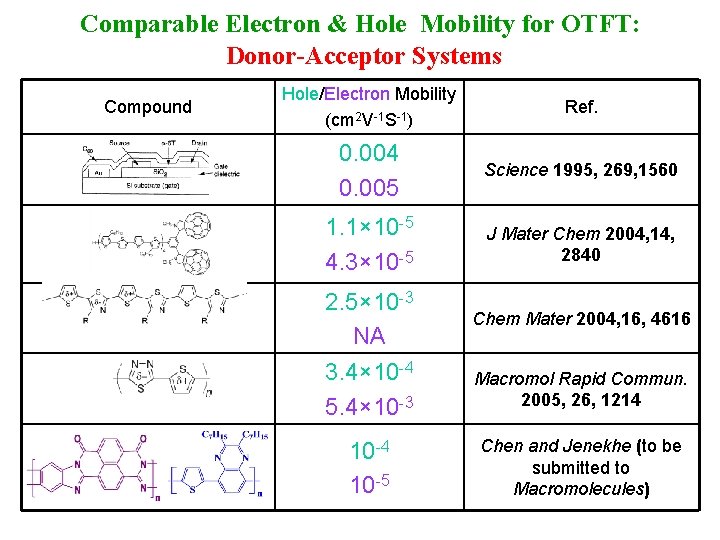
Comparable Electron & Hole Mobility for OTFT: Donor-Acceptor Systems Compound Hole/Electron Mobility (cm 2 V-1 S-1) Ref. 0. 004 0. 005 Science 1995, 269, 1560 1. 1× 10 -5 4. 3× 10 -5 J Mater Chem 2004, 14, 2840 2. 5× 10 -3 NA Chem Mater 2004, 16, 4616 3. 4× 10 -4 5. 4× 10 -3 Macromol Rapid Commun. 2005, 26, 1214 10 -5 Chen and Jenekhe (to be submitted to Macromolecules)

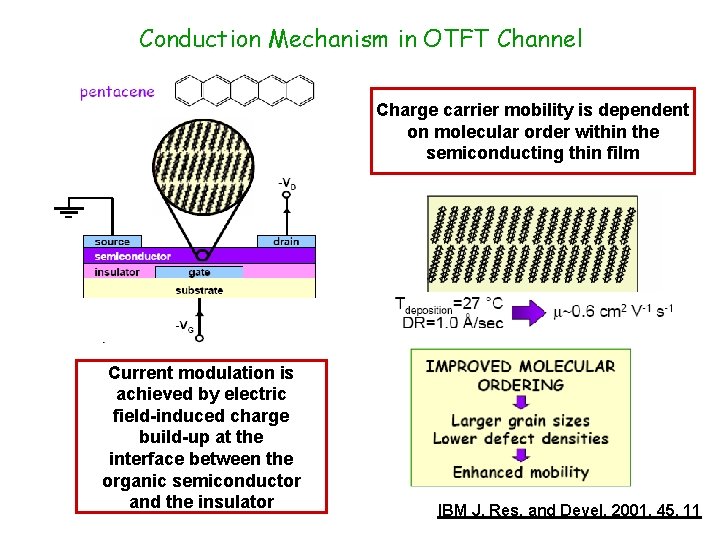
Conduction Mechanism in OTFT Channel Charge carrier mobility is dependent on molecular order within the semiconducting thin film Current modulation is achieved by electric field-induced charge build-up at the interface between the organic semiconductor and the insulator IBM J. Res. and Devel. 2001, 45, 11

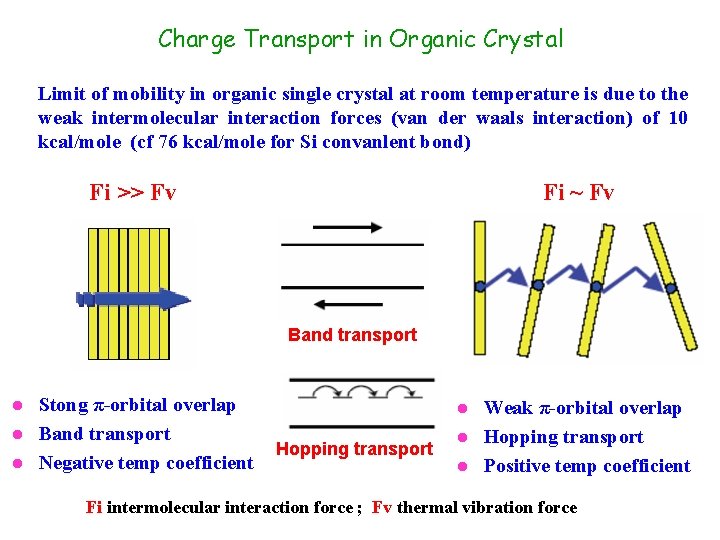
Charge Transport in Organic Crystal Limit of mobility in organic single crystal at room temperature is due to the weak intermolecular interaction forces (van der waals interaction) of 10 kcal/mole (cf 76 kcal/mole for Si convanlent bond) Fi >> Fv Fi ~ Fv Band transport Stong π-orbital overlap l Band transport l Negative temp coefficient l Weak π-orbital overlap l Hopping transport l Positive temp coefficient l Hopping transport Fi intermolecular interaction force ; Fv thermal vibration force

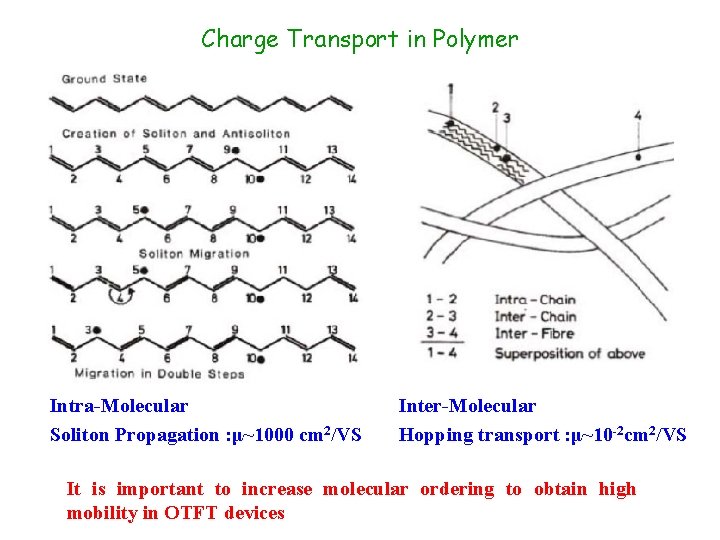
Charge Transport in Polymer Intra-Molecular Soliton Propagation : μ~1000 cm 2/VS Inter-Molecular Hopping transport : μ~10 -2 cm 2/VS It is important to increase molecular ordering to obtain high mobility in OTFT devices

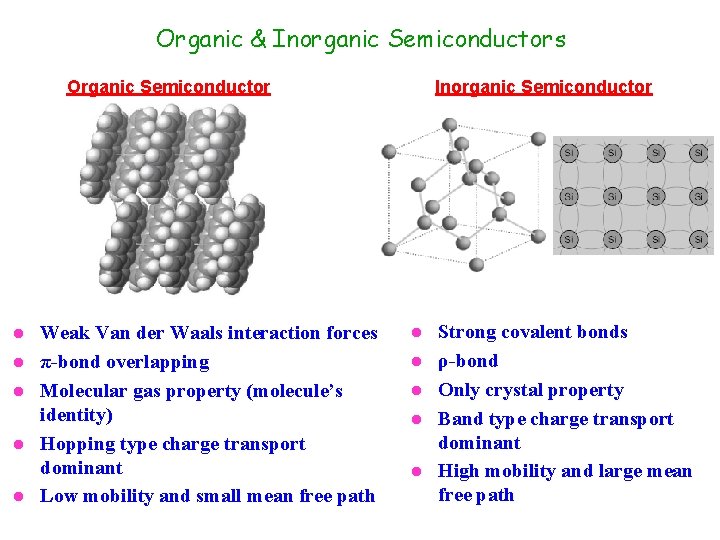
Organic & Inorganic Semiconductors Organic Semiconductor l l l Weak Van der Waals interaction forces π-bond overlapping Molecular gas property (molecule’s identity) Hopping type charge transport dominant Low mobility and small mean free path Inorganic Semiconductor l l l Strong covalent bonds ρ-bond Only crystal property Band type charge transport dominant High mobility and large mean free path

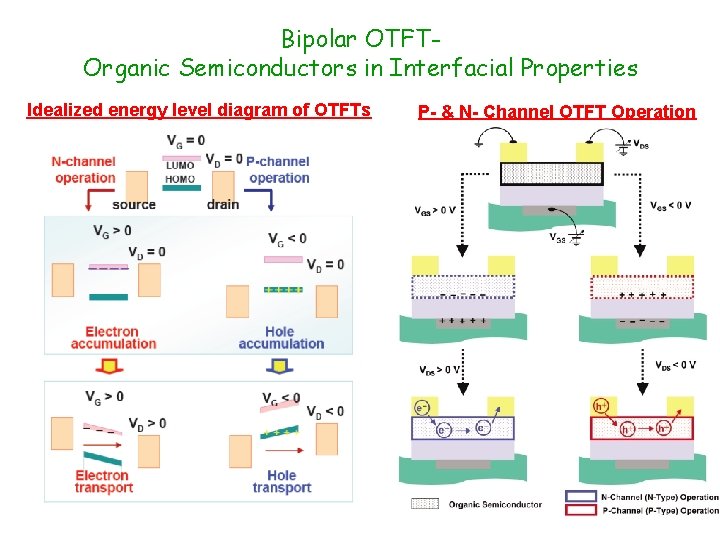
Bipolar OTFTOrganic Semiconductors in Interfacial Properties Idealized energy level diagram of OTFTs P- & N- Channel OTFT Operation

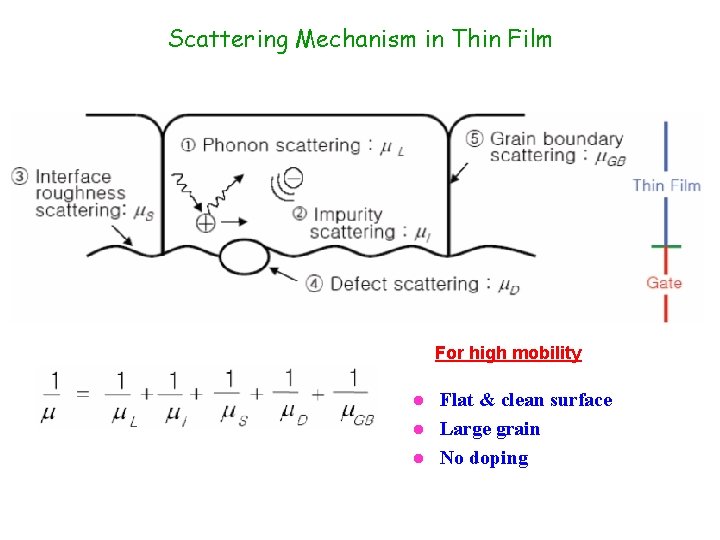
Scattering Mechanism in Thin Film For high mobility Flat & clean surface l Large grain l No doping l

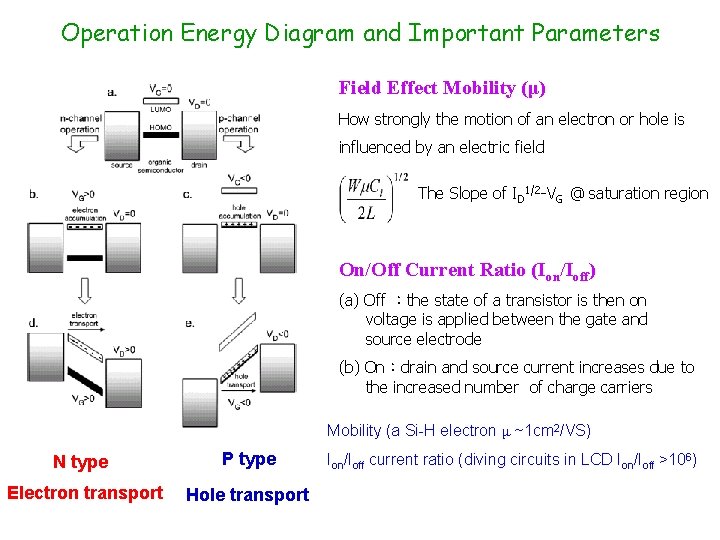
Operation Energy Diagram and Important Parameters Field Effect Mobility (μ) How strongly the motion of an electron or hole is influenced by an electric field The Slope of ID 1/2 -VG @ saturation region On/Off Current Ratio (Ion/Ioff) (a) Off :the state of a transistor is then on voltage is applied between the gate and source electrode (b) On:drain and source current increases due to the increased number of charge carriers Mobility (a Si-H electron μ ~1 cm 2/VS) N type P type Electron transport Hole transport Ion/Ioff current ratio (diving circuits in LCD Ion/Ioff >106)

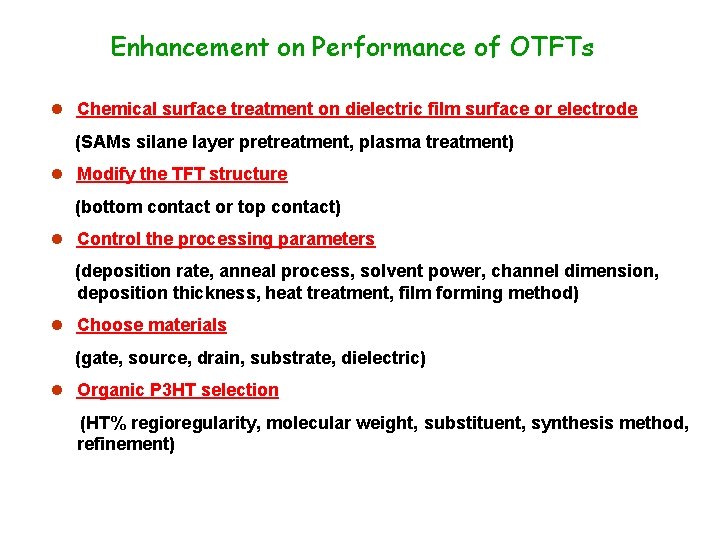
Enhancement on Performance of OTFTs l Chemical surface treatment on dielectric film surface or electrode (SAMs silane layer pretreatment, plasma treatment) l Modify the TFT structure (bottom contact or top contact) l Control the processing parameters (deposition rate, anneal process, solvent power, channel dimension, deposition thickness, heat treatment, film forming method) l Choose materials (gate, source, drain, substrate, dielectric) l Organic P 3 HT selection (HT% regioregularity, molecular weight, substituent, synthesis method, refinement)

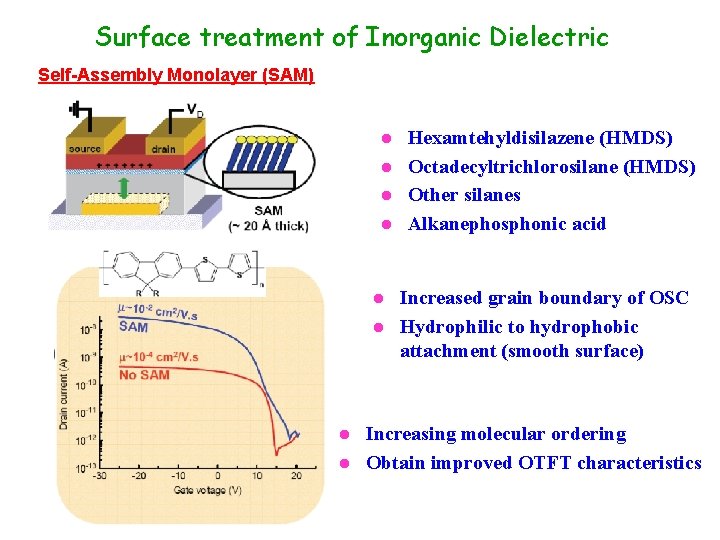
Surface treatment of Inorganic Dielectric Self-Assembly Monolayer (SAM) Hexamtehyldisilazene (HMDS) l Octadecyltrichlorosilane (HMDS) l Other silanes l Alkanephosphonic acid l Increased grain boundary of OSC l Hydrophilic to hydrophobic attachment (smooth surface) l Increasing molecular ordering l Obtain improved OTFT characteristics l

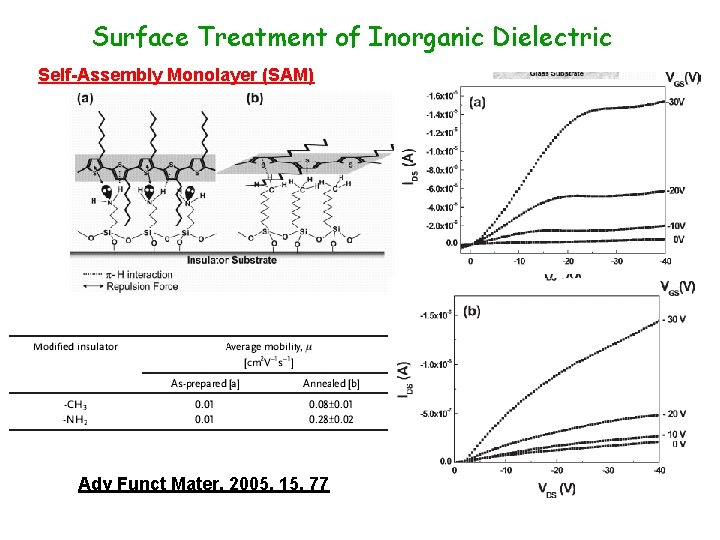
Surface Treatment of Inorganic Dielectric Self-Assembly Monolayer (SAM) Adv Funct Mater, 2005, 15, 77

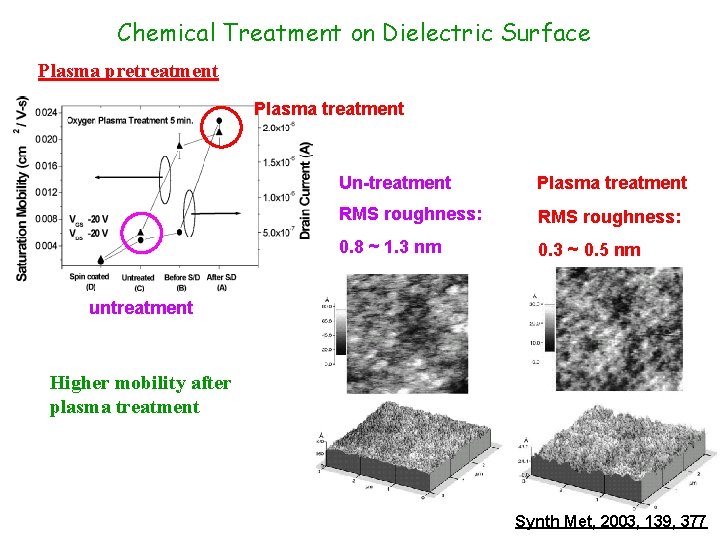
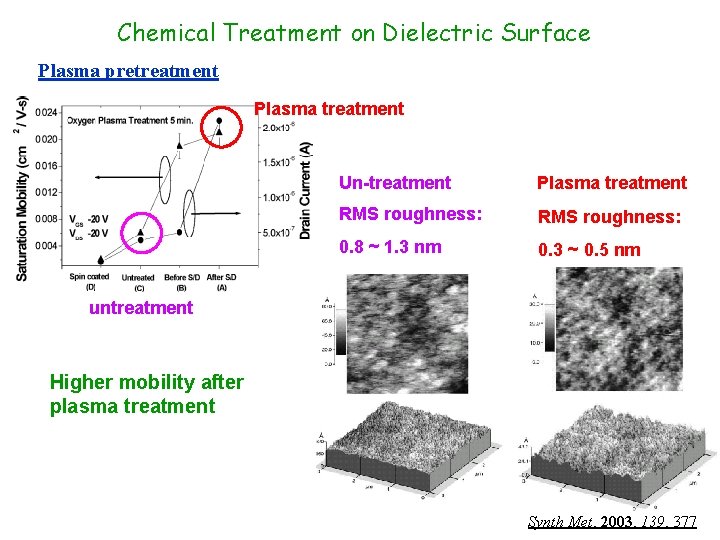
Chemical Treatment on Dielectric Surface Plasma pretreatment Plasma treatment Un-treatment Plasma treatment RMS roughness: 0. 8 ~ 1. 3 nm 0. 3 ~ 0. 5 nm untreatment Higher mobility after plasma treatment Synth Met, 2003, 139, 377

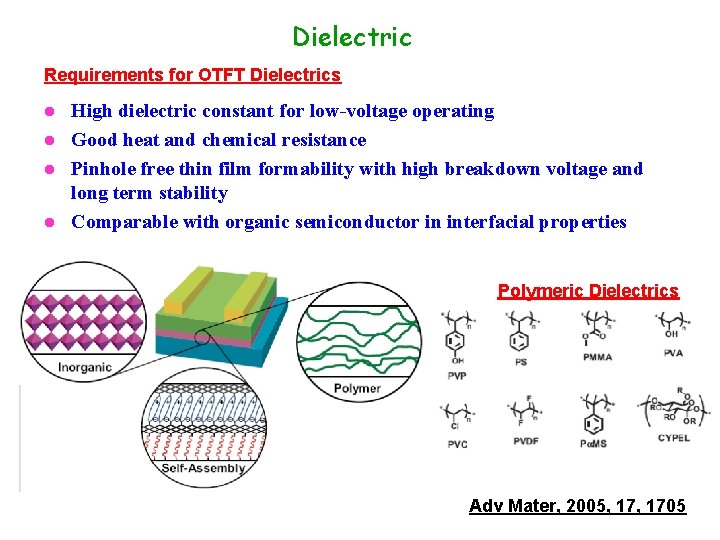
Dielectric Requirements for OTFT Dielectrics High dielectric constant for low-voltage operating l Good heat and chemical resistance l Pinhole free thin film formability with high breakdown voltage and long term stability l Comparable with organic semiconductor in interfacial properties l Polymeric Dielectrics Adv Mater, 2005, 1705

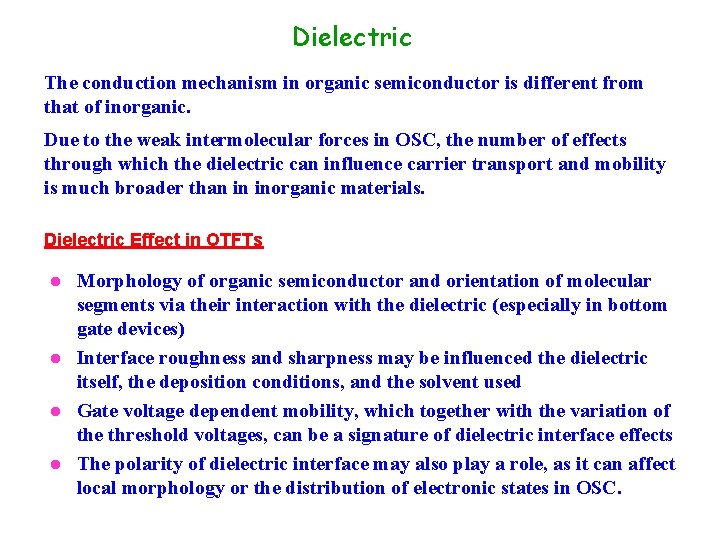
Dielectric The conduction mechanism in organic semiconductor is different from that of inorganic. Due to the weak intermolecular forces in OSC, the number of effects through which the dielectric can influence carrier transport and mobility is much broader than in inorganic materials. Dielectric Effect in OTFTs Morphology of organic semiconductor and orientation of molecular segments via their interaction with the dielectric (especially in bottom gate devices) l Interface roughness and sharpness may be influenced the dielectric itself, the deposition conditions, and the solvent used l Gate voltage dependent mobility, which together with the variation of the threshold voltages, can be a signature of dielectric interface effects l The polarity of dielectric interface may also play a role, as it can affect local morphology or the distribution of electronic states in OSC. l

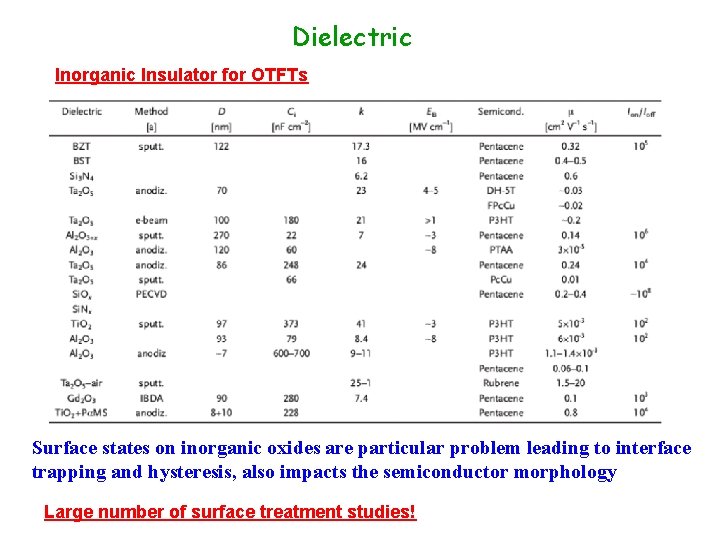
Dielectric Inorganic Insulator for OTFTs Surface states on inorganic oxides are particular problem leading to interface trapping and hysteresis, also impacts the semiconductor morphology Large number of surface treatment studies!

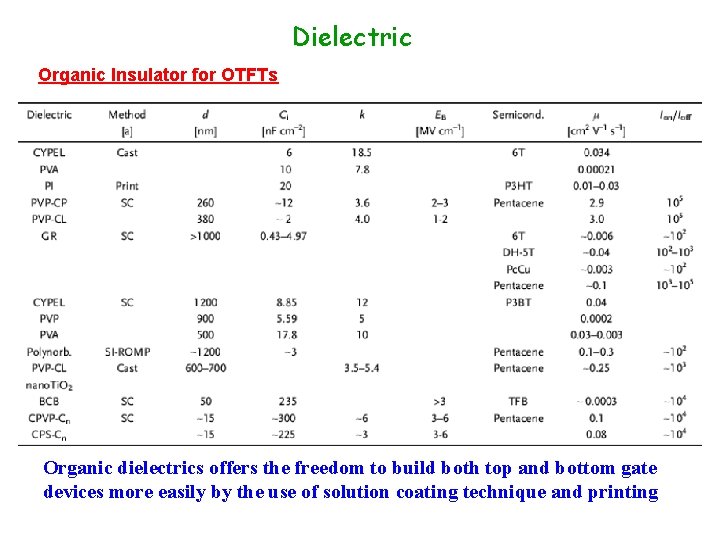
Dielectric Organic Insulator for OTFTs Organic dielectrics offers the freedom to build both top and bottom gate devices more easily by the use of solution coating technique and printing

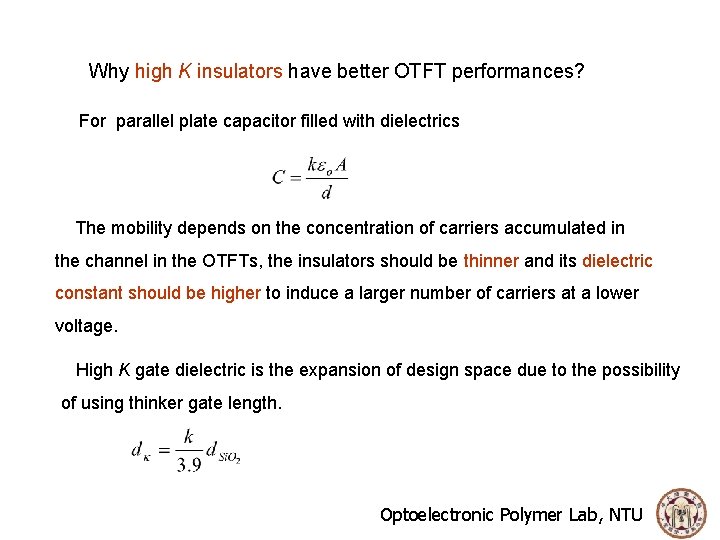
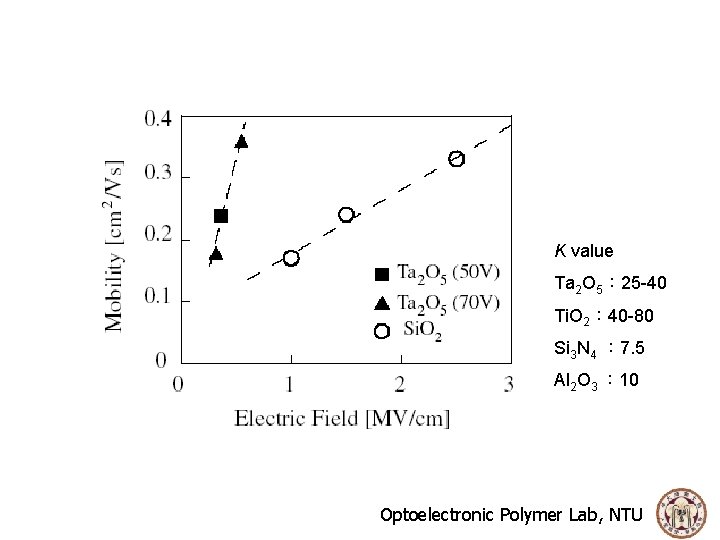
Why high K insulators have better OTFT performances? For parallel plate capacitor filled with dielectrics The mobility depends on the concentration of carriers accumulated in the channel in the OTFTs, the insulators should be thinner and its dielectric constant should be higher to induce a larger number of carriers at a lower voltage. High K gate dielectric is the expansion of design space due to the possibility of using thinker gate length. Optoelectronic Polymer Lab, NTU

K value Ta 2 O 5: 25 -40 Ti. O 2: 40 -80 Si 3 N 4 : 7. 5 Al 2 O 3 : 10 Optoelectronic Polymer Lab, NTU

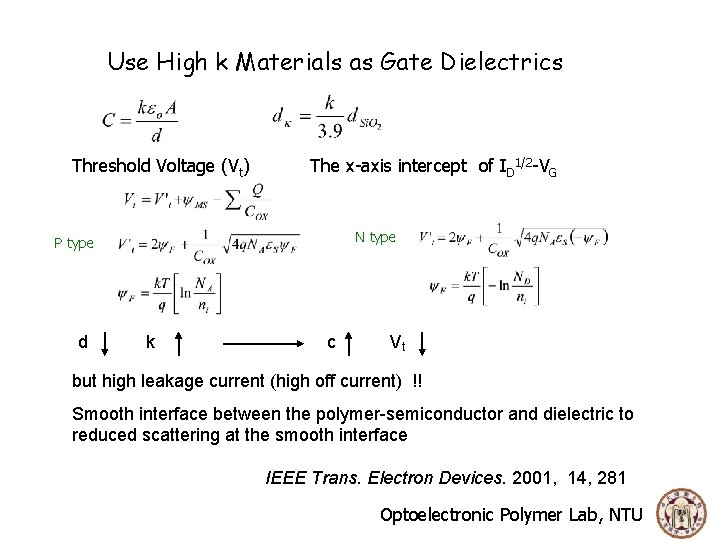
Use High k Materials as Gate Dielectrics Threshold Voltage (Vt) The x-axis intercept of ID 1/2 -VG N type P type d k c Vt but high leakage current (high off current) !! Smooth interface between the polymer-semiconductor and dielectric to reduced scattering at the smooth interface IEEE Trans. Electron Devices. 2001, 14, 281 Optoelectronic Polymer Lab, NTU

Why choosing Organic materials as insulators? The drawbacks of using inorganic materials as insulators:Difficulty on building electronic devices on plastic substrate; High processing temperature、adhesion to substrate、processing method、Cost、 large area?

Organic Polymers

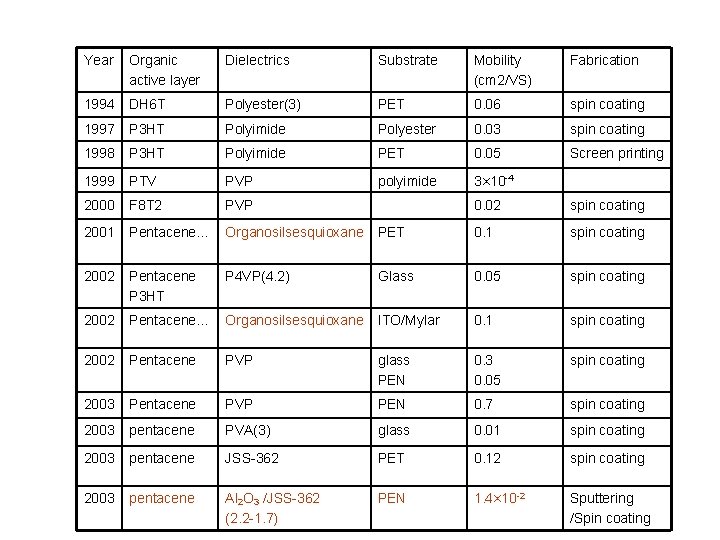
Year Organic active layer Dielectrics Substrate Mobility (cm 2/VS) Fabrication 1994 DH 6 T Polyester(3) PET 0. 06 spin coating 1997 P 3 HT Polyimide Polyester 0. 03 spin coating 1998 P 3 HT Polyimide PET 0. 05 Screen printing 1999 PTV PVP polyimide 3× 10 -4 2000 F 8 T 2 PVP 2001 Pentacene… Organosilsesquioxane 2002 Pentacene P 3 HT 2002 0. 02 spin coating PET 0. 1 spin coating P 4 VP(4. 2) Glass 0. 05 spin coating Pentacene… Organosilsesquioxane ITO/Mylar 0. 1 spin coating 2002 Pentacene PVP glass PEN 0. 3 0. 05 spin coating 2003 Pentacene PVP PEN 0. 7 spin coating 2003 pentacene PVA(3) glass 0. 01 spin coating 2003 pentacene JSS-362 PET 0. 12 spin coating 2003 pentacene Al 2 O 3 /JSS-362 (2. 2 -1. 7) PEN 1. 4× 10 -2 Sputtering /Spin coating

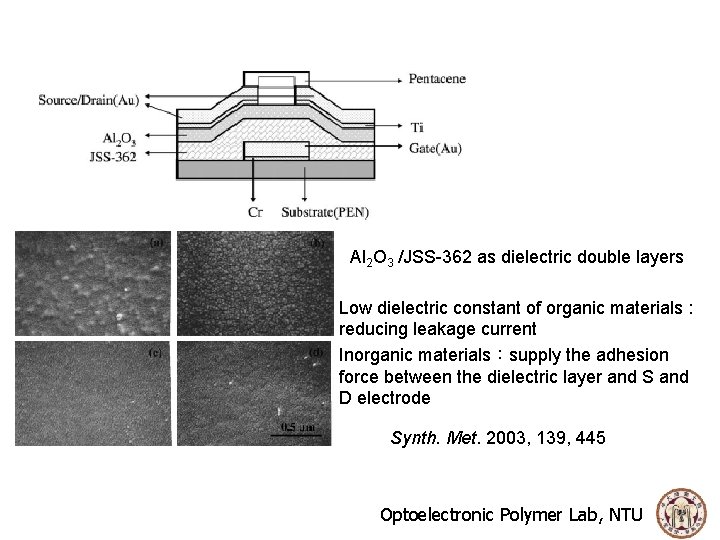
Al 2 O 3 /JSS-362 as dielectric double layers Low dielectric constant of organic materials : reducing leakage current Inorganic materials:supply the adhesion force between the dielectric layer and S and D electrode Synth. Met. 2003, 139, 445 Optoelectronic Polymer Lab, NTU

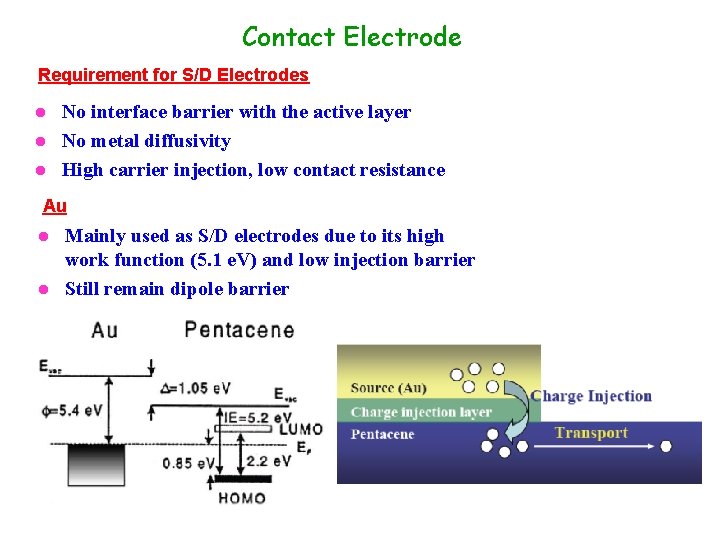
Contact Electrode Requirement for S/D Electrodes No interface barrier with the active layer l No metal diffusivity l High carrier injection, low contact resistance l Au Mainly used as S/D electrodes due to its high work function (5. 1 e. V) and low injection barrier l Still remain dipole barrier l

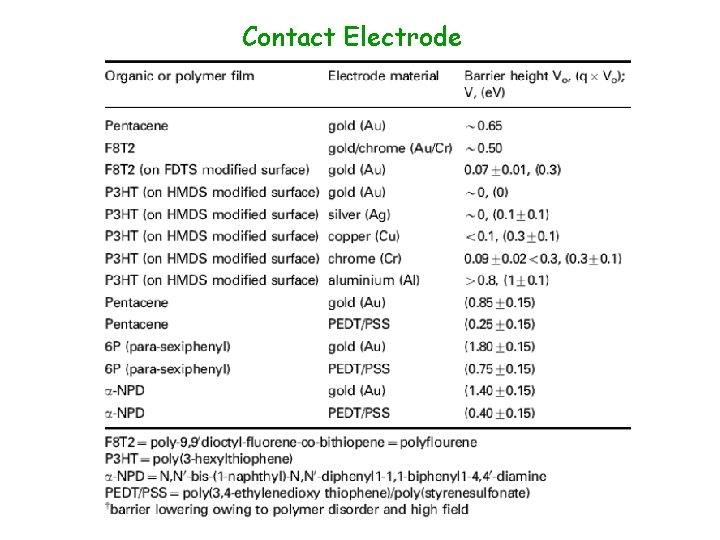
Contact Electrode

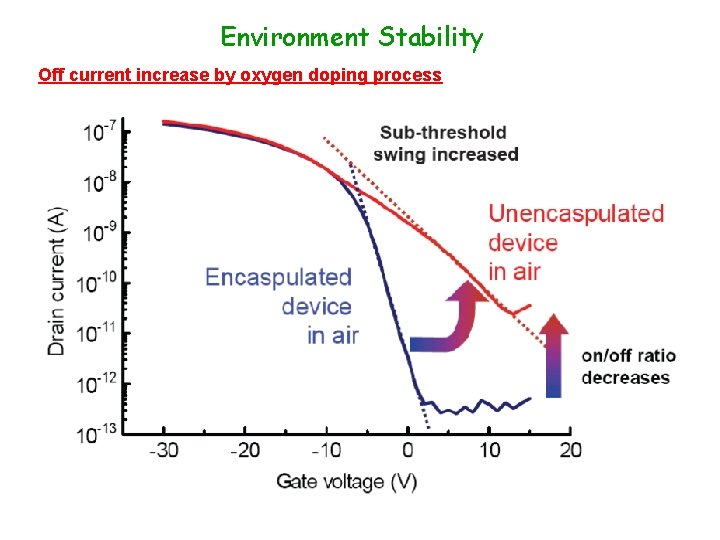
Environment Stability Off current increase by oxygen doping process

Improvement of P 3 HT OTFTs l Chemical surface treatment on dielectric film surface or electrode (SAMs silane layer pretreatment, plasma treatment) l Modify the TFT structure (bottom contact or top contact) l Control the processing parameters (deposition rate, anneal process, solvent power, channel dimension, deposition thickness, heat treatment, film forming method) l Choose materials (gate, source, drain, substrate, dielectric) l Organic P 3 HT selection (HT% regioregularity, molecular weight, substituent, synthesis method, refinement)

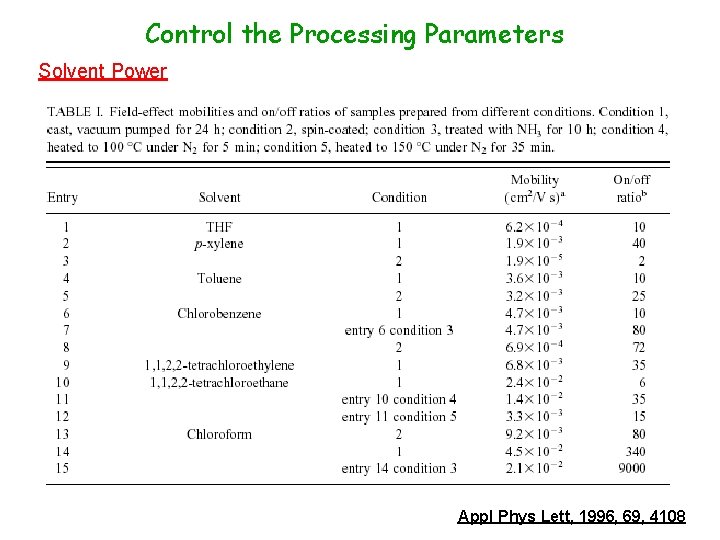
Control the Processing Parameters Solvent Power Appl Phys Lett, 1996, 69, 4108

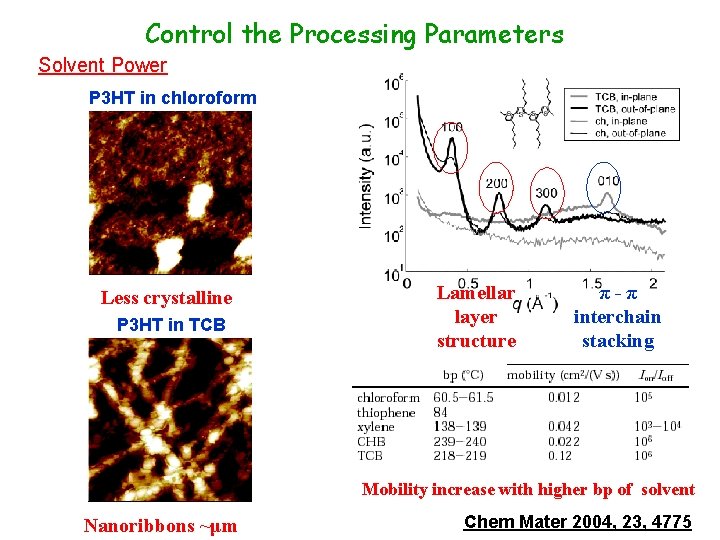
Control the Processing Parameters Solvent Power P 3 HT in chloroform Less crystalline P 3 HT in TCB Lamellar layer structure π-π interchain stacking Mobility increase with higher bp of solvent Nanoribbons ~μm Chem Mater 2004, 23, 4775

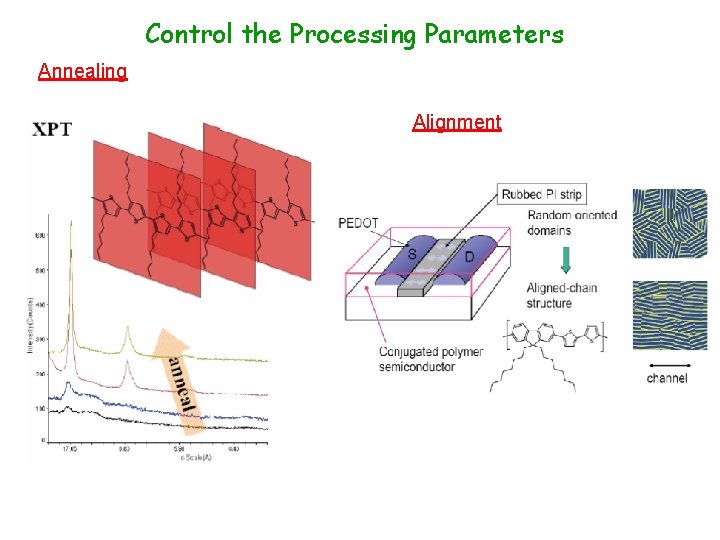
Control the Processing Parameters Annealing Alignment

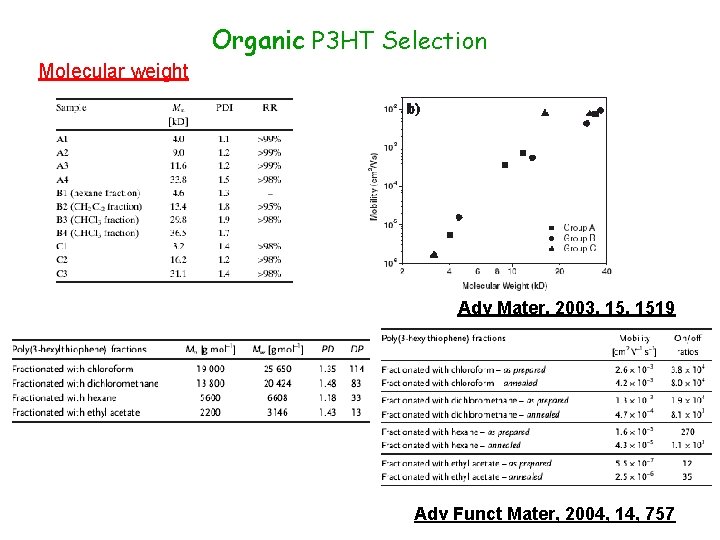
Organic P 3 HT Selection Molecular weight Adv Mater, 2003, 1519 Adv Funct Mater, 2004, 14, 757

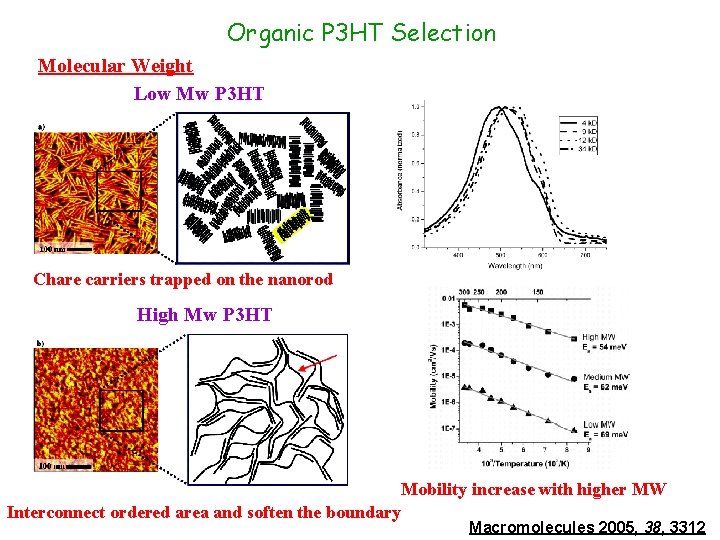
Organic P 3 HT Selection Molecular Weight Low Mw P 3 HT Chare carriers trapped on the nanorod High Mw P 3 HT Mobility increase with higher MW Interconnect ordered area and soften the boundary Macromolecules 2005, 38, 3312

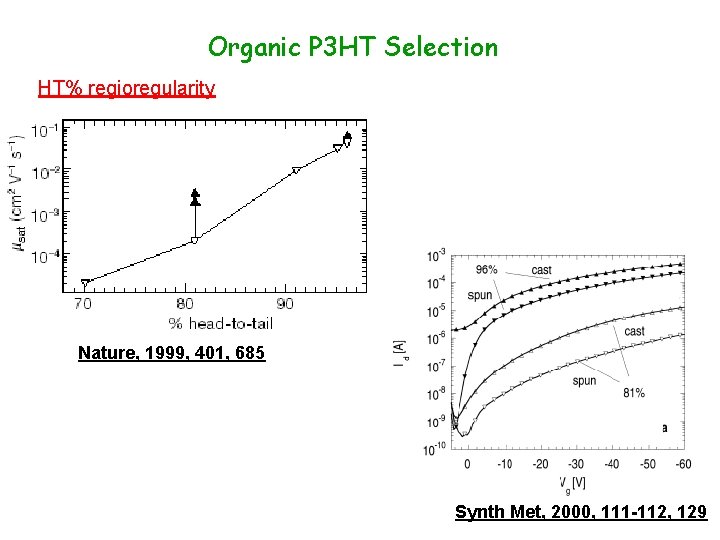
Organic P 3 HT Selection HT% regioregularity Nature, 1999, 401, 685 Synth Met, 2000, 111 -112, 129

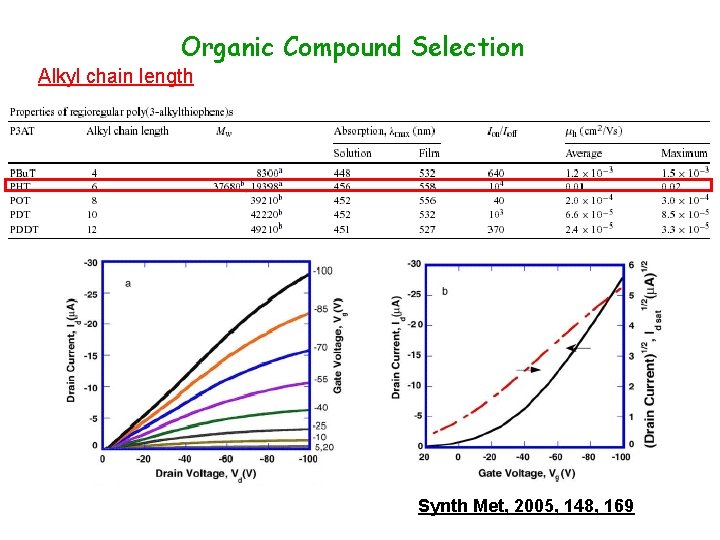
Organic Compound Selection Alkyl chain length Synth Met, 2005, 148, 169

Chemical Treatment on Dielectric Surface Plasma pretreatment Plasma treatment Un-treatment Plasma treatment RMS roughness: 0. 8 ~ 1. 3 nm 0. 3 ~ 0. 5 nm untreatment Higher mobility after plasma treatment Synth Met, 2003, 139, 377

Semiconductor Deposition Methods Organic semiconductors are deposited either from vapor or solution phase depending on their vapor pressure and solubility Device performance of OTFTs is greatly influenced by various deposition conditions due to the different resulting molecular structure and thin film morphology

How to Get High Mobility ? Ways of Mobility Improvement Homo/LUMO of the individual molecules must be at levels where hole/electrons can be induced at accessible applied electric fields. l The solid should be extremely pure since impurities act as charge carrier traps. l The molecules should be preferentially oriented with the long axes approximately parallel to the substrate since most efficient charge transport occurs along the direction of intermolecular ππstacking l The crsytalline domains of the semiconductor must cover the area between the S and D contacts uniformly. l

Reference l G. Horowitz, Adv. Mater. 2000, 14, 365 l Katz, H. E. ; Bao, Z. , J. Phys. Chem. B. , 2000, 104, 671 l Dimitrakopoulous, C. D. ; Mascaro, D. J. , IBM J. Res. & Dev. 2001, 45, 11 l Katz, H. E. ; Bao, Z. ; Gilat, S. L. , Acc. Chem. Res. , 2001, 34, 359 l Dimitrakopoulous, C. D. ; Malenfant, D. R. L. Adv. Mater. 2002, 14, 99 l Horowitz, G. J. Mater. Res. 2004, 1946 l Newman, C. R. ; Frisbie, C. D. ; da silva Filho, D. A. ; Bredas, J. L. ; P. C. Ewbank, Mann K. R. Chem. Mater. 2004, 16, 4436 l Veres, J. ; Ogier, S. ; Lloyd, G. Chem. Mater. 2004, 16, 4543 l Ling, M. M. ; Bao, Z. Chem. Mater. 2004, 16, 4824 l Chua, L. L. ; Zaumsell, J. ; Chang, J. F. ; Ou, E. C. W. ; Ho, P. K. H. ; Sirringhaus, H. ; Friend, R. H. Nature, 2005, 434, 194 l Sun, Y. ; Liu, Y. ; Zhu, D. J. Mater. Chem. 2005, 15, 53 l Facchetti, A. ; Yon, M. H. ; Marks, T. J. Adv. Mater. 2005, 1705 l Sirringhaus, H. Adv. Mater. 2005, 17, 2411 l Reichmanis, E. ; Katz, H. E. ; Kloc, C. ; Maliakal, A. Bell Labs Technical J. 2005, 10, 87 l Dodabalapur, A. Materials Today 2006, 9 , 24 l Facchetti, A. Materials Today 2007, 10, 28 l Zaumseil, J. ; Sirringhaus, H. Chem. Rev. 2007, 1296