Electron transport Chemiosmotic Theory Electron Transport Electrons carried





- Slides: 48

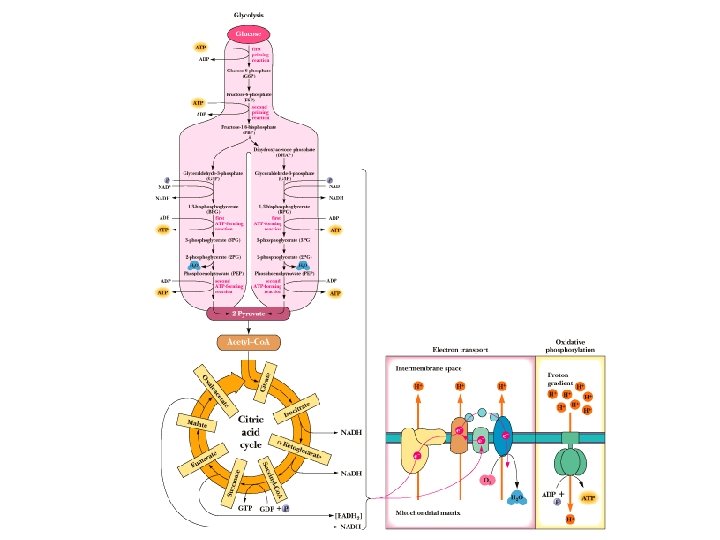
Electron transport


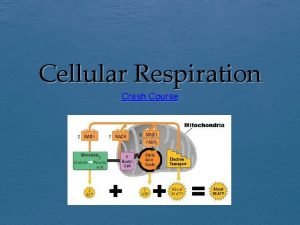
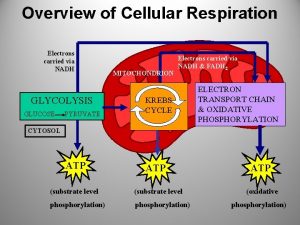
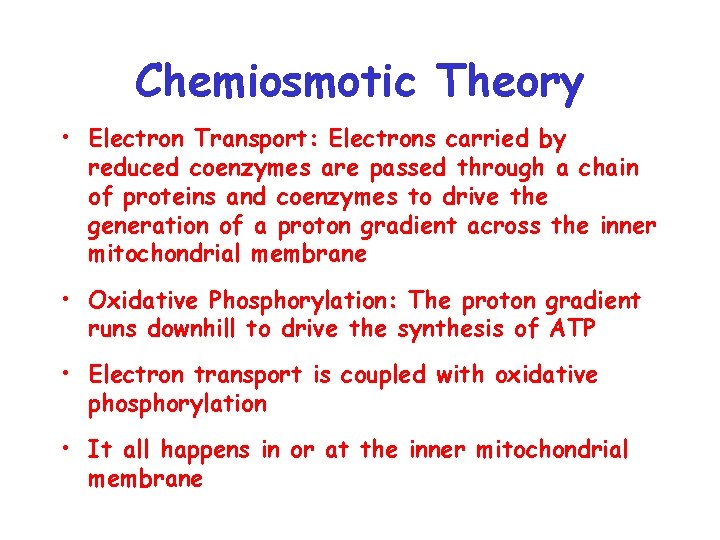
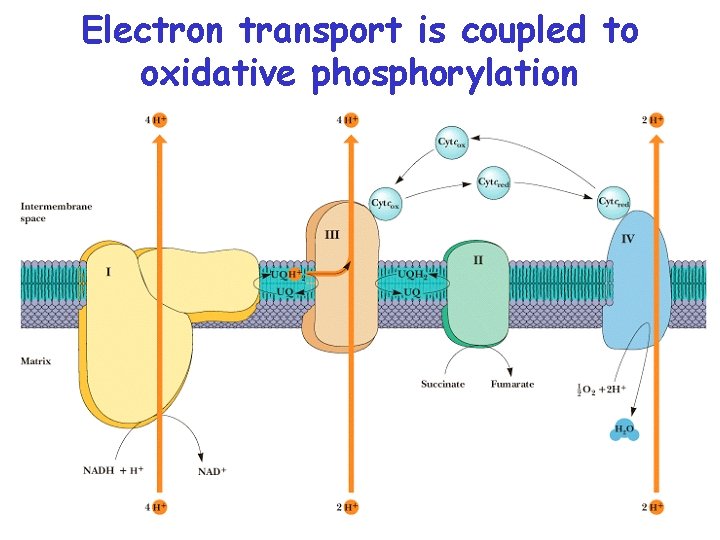
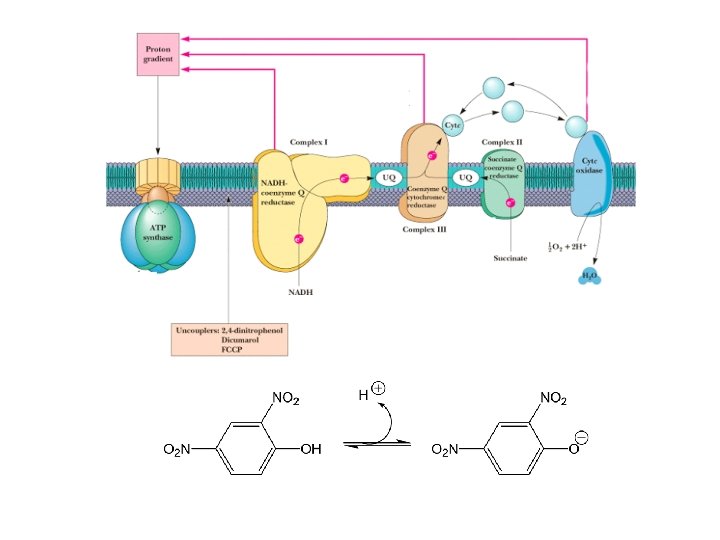
Chemiosmotic Theory • Electron Transport: Electrons carried by reduced coenzymes are passed through a chain of proteins and coenzymes to drive the generation of a proton gradient across the inner mitochondrial membrane • Oxidative Phosphorylation: The proton gradient runs downhill to drive the synthesis of ATP • Electron transport is coupled with oxidative phosphorylation • It all happens in or at the inner mitochondrial membrane

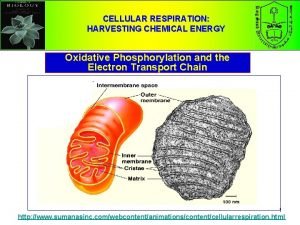
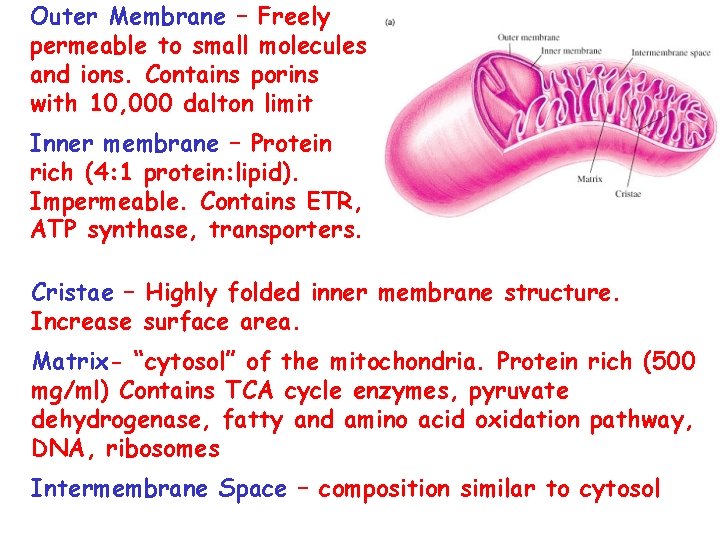
Outer Membrane – Freely permeable to small molecules and ions. Contains porins with 10, 000 dalton limit Inner membrane – Protein rich (4: 1 protein: lipid). Impermeable. Contains ETR, ATP synthase, transporters. Cristae – Highly folded inner membrane structure. Increase surface area. Matrix- “cytosol” of the mitochondria. Protein rich (500 mg/ml) Contains TCA cycle enzymes, pyruvate dehydrogenase, fatty and amino acid oxidation pathway, DNA, ribosomes Intermembrane Space – composition similar to cytosol


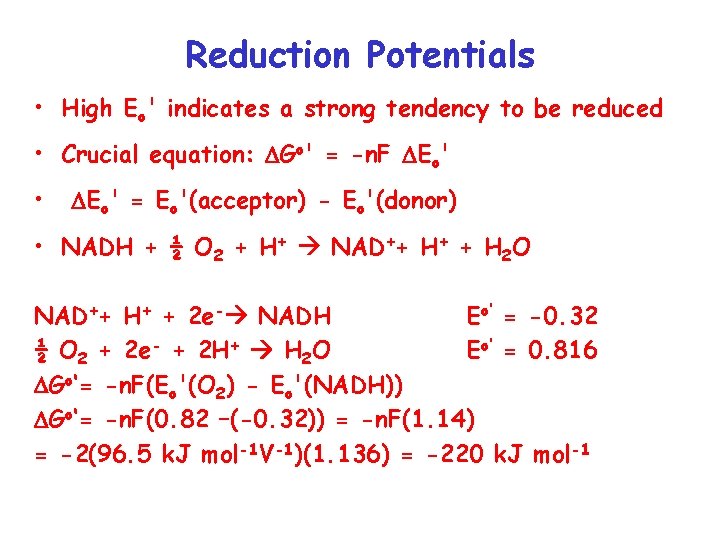
Reduction Potentials • High Eo' indicates a strong tendency to be reduced • Crucial equation: Go' = -n. F Eo' • Eo' = Eo'(acceptor) - Eo'(donor) • NADH + ½ O 2 + H+ NAD++ H+ + H 2 O NAD++ H+ + 2 e- NADH Eo’ = -0. 32 ½ O 2 + 2 e- + 2 H+ H 2 O Eo’ = 0. 816 Go‘= -n. F(Eo'(O 2) - Eo'(NADH)) Go‘= -n. F(0. 82 –(-0. 32)) = -n. F(1. 14) = -2(96. 5 k. J mol-1 V-1)(1. 136) = -220 k. J mol-1

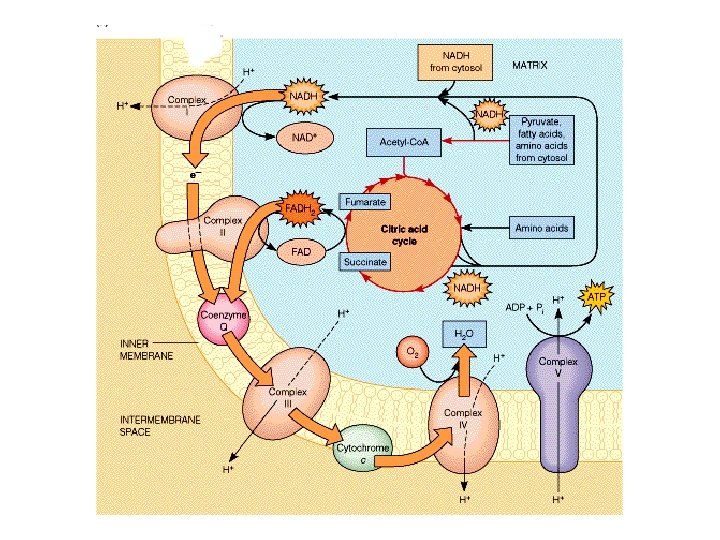
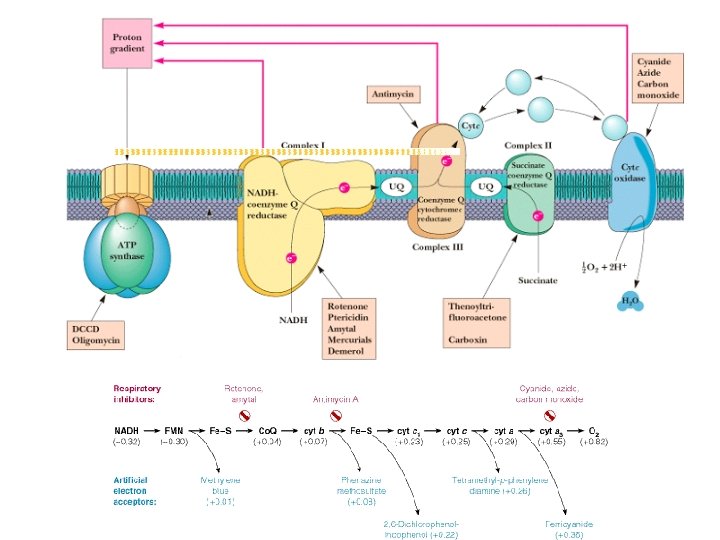
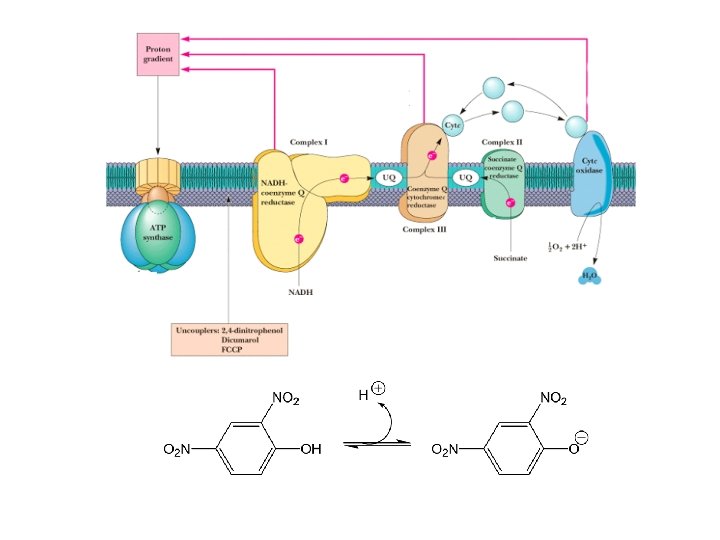
Electron Transport • Four protein complexes in the inner mitochondrial membrane • A lipid soluble coenzyme (UQ, Co. Q) and a water soluble protein (cyt c) shuttle between protein complexes • Electrons generally fall in energy through the chain - from complexes I and II to complex IV

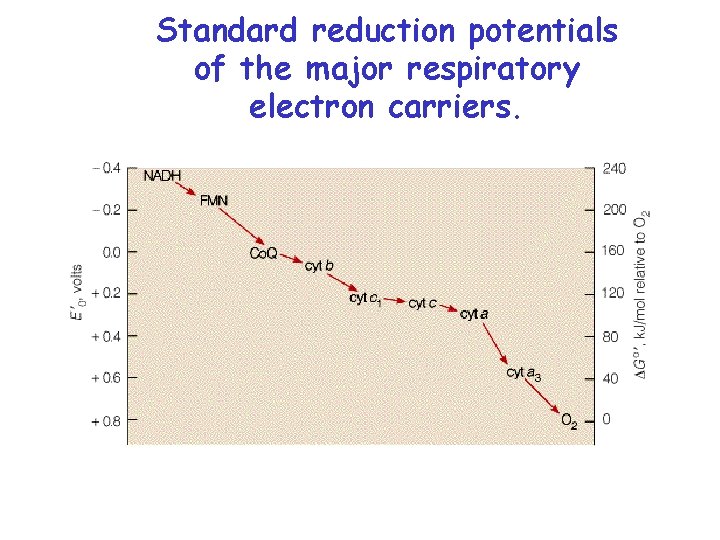
Standard reduction potentials of the major respiratory electron carriers.


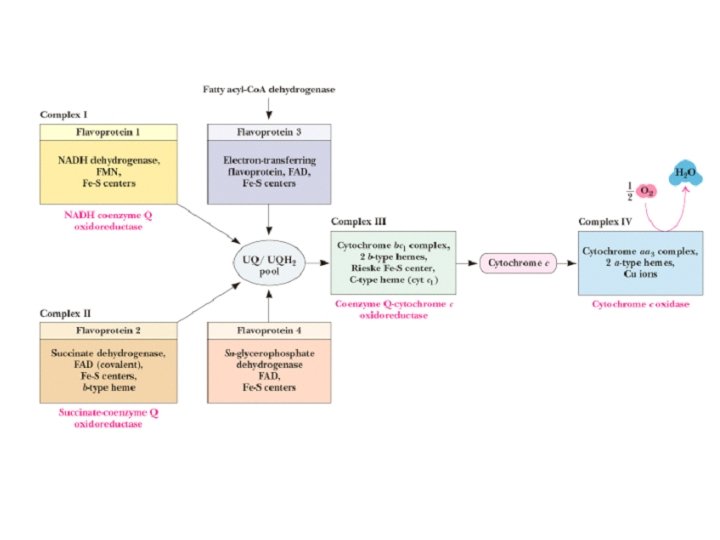
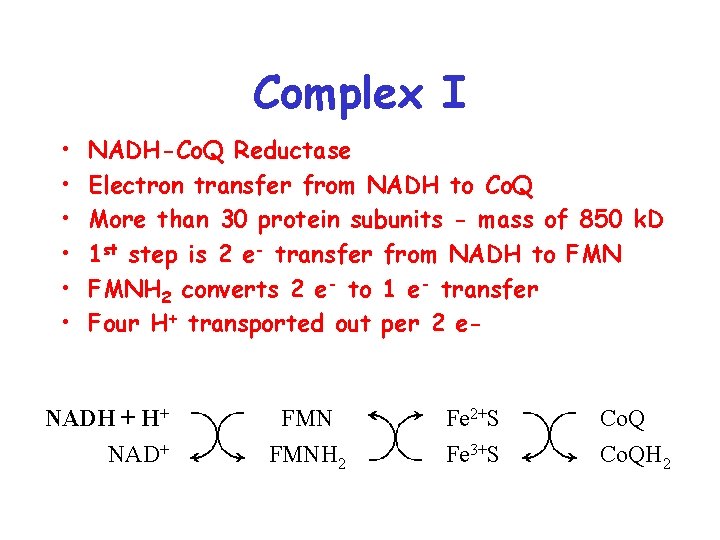
Complex I • • • NADH-Co. Q Reductase Electron transfer from NADH to Co. Q More than 30 protein subunits - mass of 850 k. D 1 st step is 2 e- transfer from NADH to FMNH 2 converts 2 e- to 1 e- transfer Four H+ transported out per 2 e- NADH + H+ NAD+ FMNH 2 Fe 2+S Fe 3+S Co. QH 2

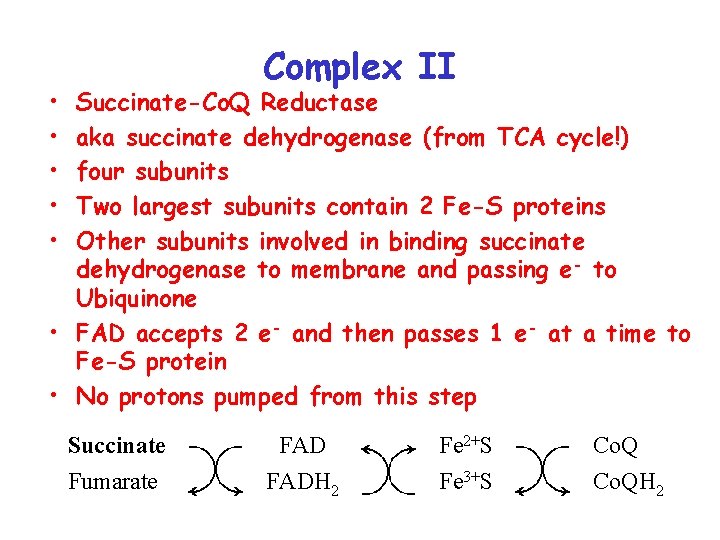
Complex II • • • Succinate-Co. Q Reductase aka succinate dehydrogenase (from TCA cycle!) four subunits Two largest subunits contain 2 Fe-S proteins Other subunits involved in binding succinate dehydrogenase to membrane and passing e- to Ubiquinone • FAD accepts 2 e- and then passes 1 e- at a time to Fe-S protein • No protons pumped from this step Succinate Fumarate FAD Fe 2+S FADH 2 Fe 3+S Co. QH 2

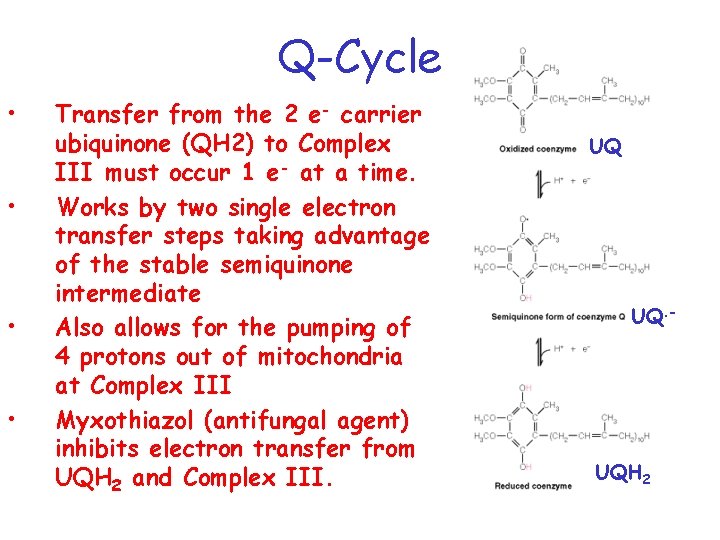
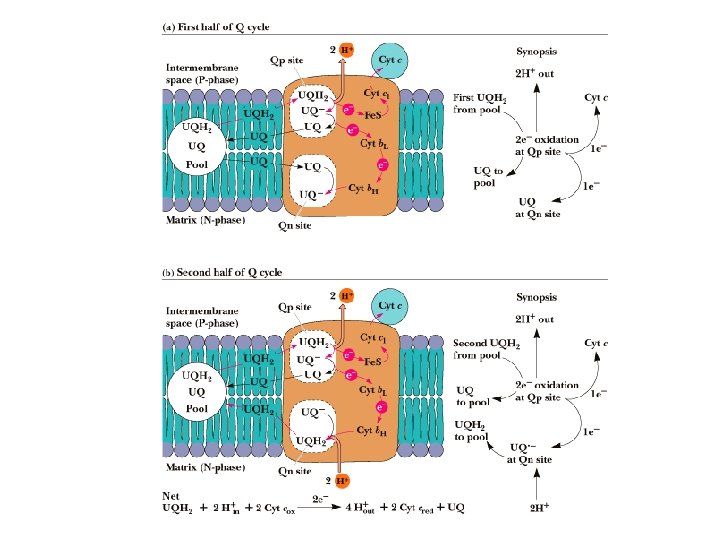
Q-Cycle • • Transfer from the 2 e- carrier ubiquinone (QH 2) to Complex III must occur 1 e- at a time. Works by two single electron transfer steps taking advantage of the stable semiquinone intermediate Also allows for the pumping of 4 protons out of mitochondria at Complex III Myxothiazol (antifungal agent) inhibits electron transfer from UQH 2 and Complex III. UQ UQ. - UQH 2


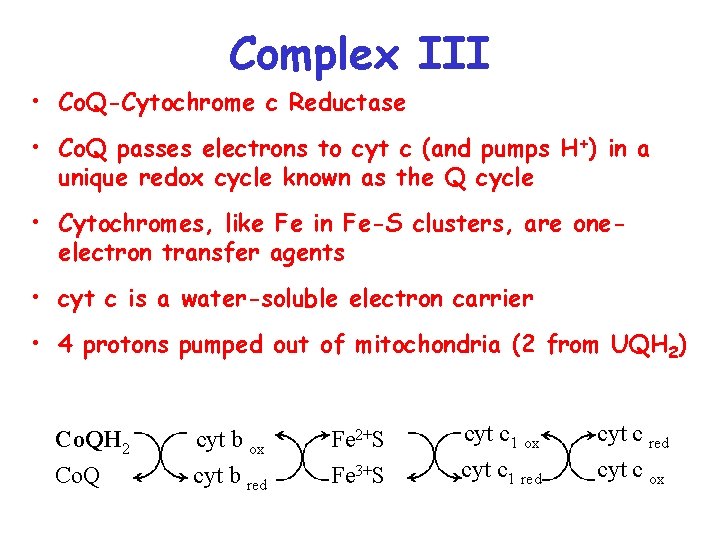
Complex III • Co. Q-Cytochrome c Reductase • Co. Q passes electrons to cyt c (and pumps H+) in a unique redox cycle known as the Q cycle • Cytochromes, like Fe in Fe-S clusters, are oneelectron transfer agents • cyt c is a water-soluble electron carrier • 4 protons pumped out of mitochondria (2 from UQH 2) Co. QH 2 Co. Q cyt b ox Fe 2+S cyt c 1 ox cyt b red Fe 3+S cyt c 1 red cyt c ox

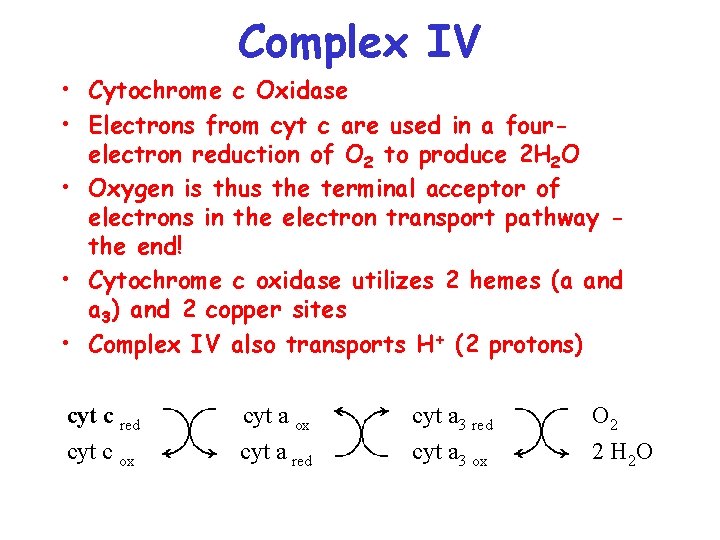
Complex IV • Cytochrome c Oxidase • Electrons from cyt c are used in a fourelectron reduction of O 2 to produce 2 H 2 O • Oxygen is thus the terminal acceptor of electrons in the electron transport pathway the end! • Cytochrome c oxidase utilizes 2 hemes (a and a 3) and 2 copper sites • Complex IV also transports H+ (2 protons) cyt c red cyt c ox cyt a red cyt a 3 ox O 2 2 H 2 O

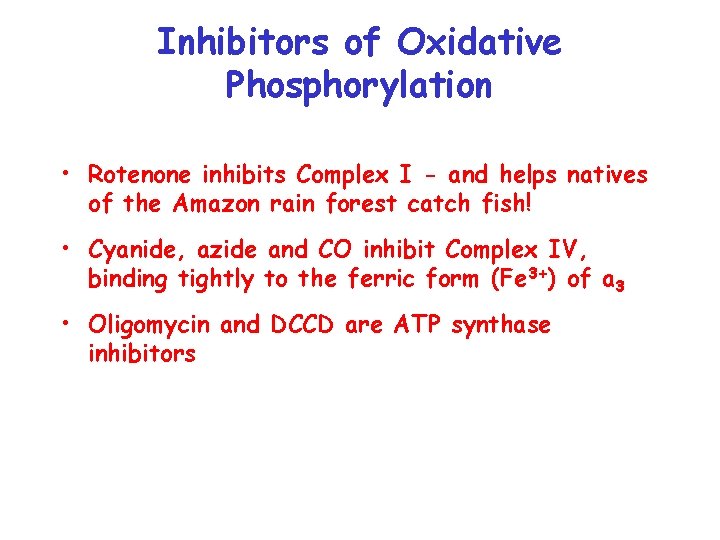
Inhibitors of Oxidative Phosphorylation • Rotenone inhibits Complex I - and helps natives of the Amazon rain forest catch fish! • Cyanide, azide and CO inhibit Complex IV, binding tightly to the ferric form (Fe 3+) of a 3 • Oligomycin and DCCD are ATP synthase inhibitors


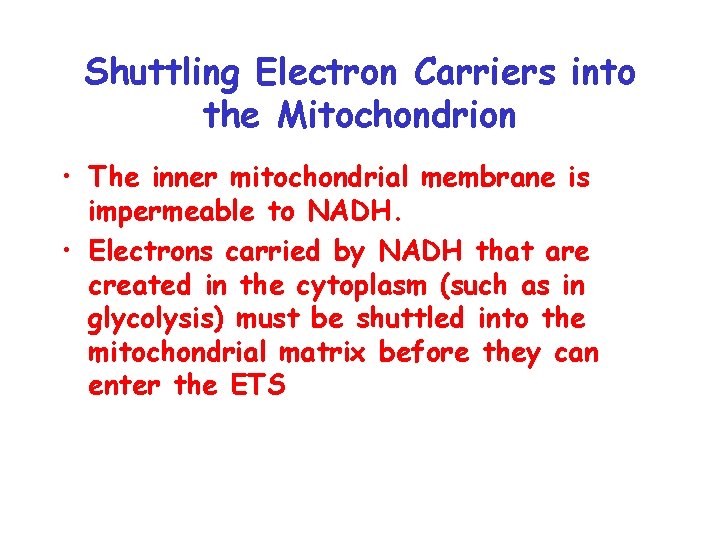
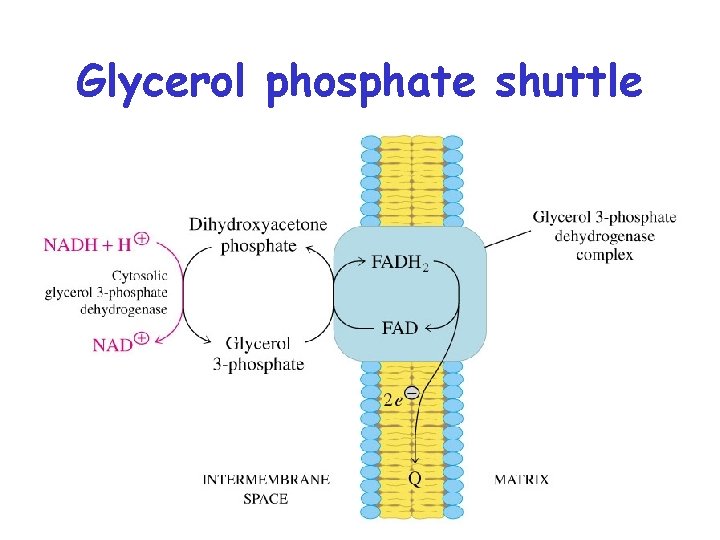
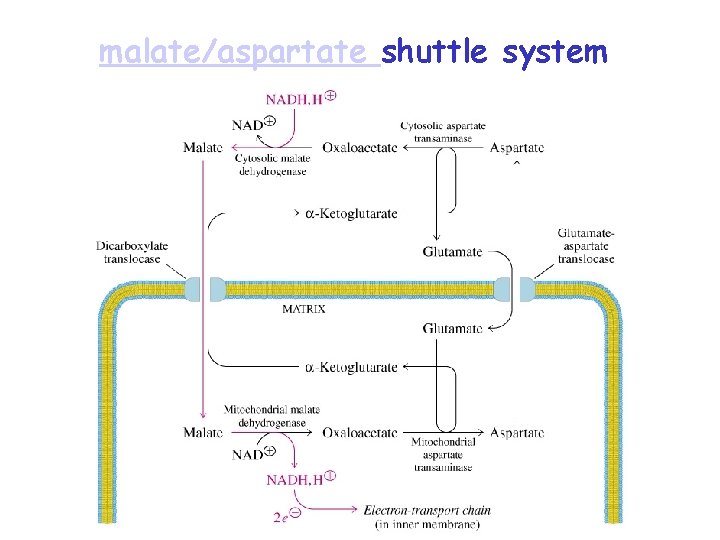
Shuttling Electron Carriers into the Mitochondrion • The inner mitochondrial membrane is impermeable to NADH. • Electrons carried by NADH that are created in the cytoplasm (such as in glycolysis) must be shuttled into the mitochondrial matrix before they can enter the ETS

Glycerol phosphate shuttle

malate/aspartate shuttle system

Electron transport is coupled to oxidative phosphorylation

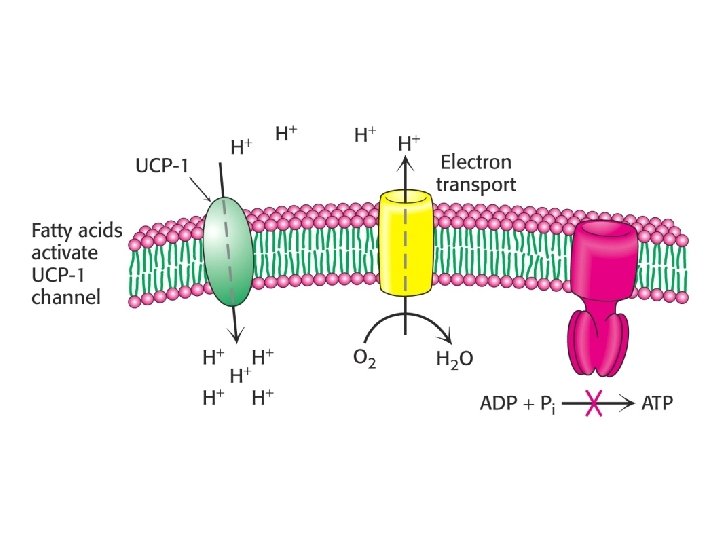
Uncouplers • Uncouplers disrupt the tight coupling between electron transport and oxidative phosphorylation by dissipating the proton gradient • Uncouplers are hydrophobic molecules with a dissociable proton • They shuttle back and forth across the membrane, carrying protons to dissipate the gradient • w/o oxidative-phosphorylation energy lost as heat • Dinitrophenol once used as diet drug, people ran 107 o. F temperatures


Oxidative phosphorylation

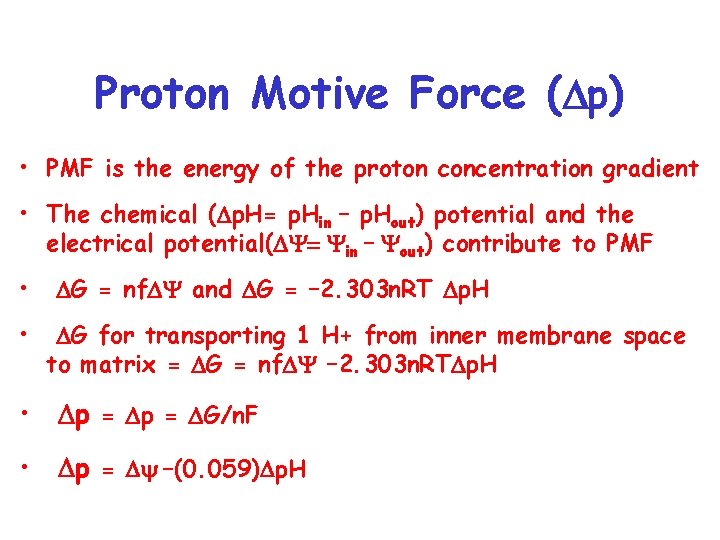
Proton Motive Force ( p) • PMF is the energy of the proton concentration gradient • The chemical ( p. H= p. Hin – p. Hout) potential and the electrical potential( Y= Yin – Yout) contribute to PMF • • G = nf Y and G = – 2. 303 n. RT p. H G for transporting 1 H+ from inner membrane space to matrix = G = nf Y – 2. 303 n. RT p. H • p = G/n. F • p = y –(0. 059) p. H

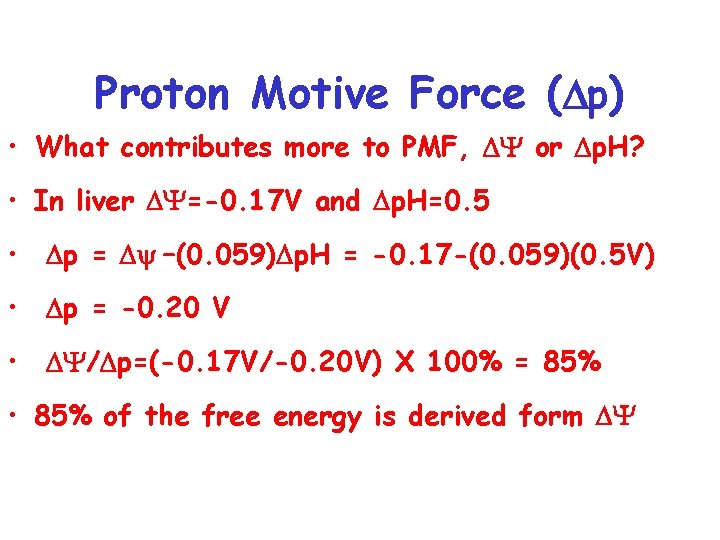
Proton Motive Force ( p) • What contributes more to PMF, Y or p. H? • In liver Y=-0. 17 V and p. H=0. 5 • p = y –(0. 059) p. H = -0. 17 -(0. 059)(0. 5 V) • p = -0. 20 V • Y/ p=(-0. 17 V/-0. 20 V) X 100% = 85% • 85% of the free energy is derived form Y

Proton Motive Force ( p) • How much free energy generated from one proton? • G = n. F P = (1)(96. 48 k. J/Vmole)(-0. 2 V) = -19 k. J/mole • To make 1 ATP need 30 k. J/mole. • Need to translocate more than one proton to make one ATP • ETC translocates 10 protons per NADH

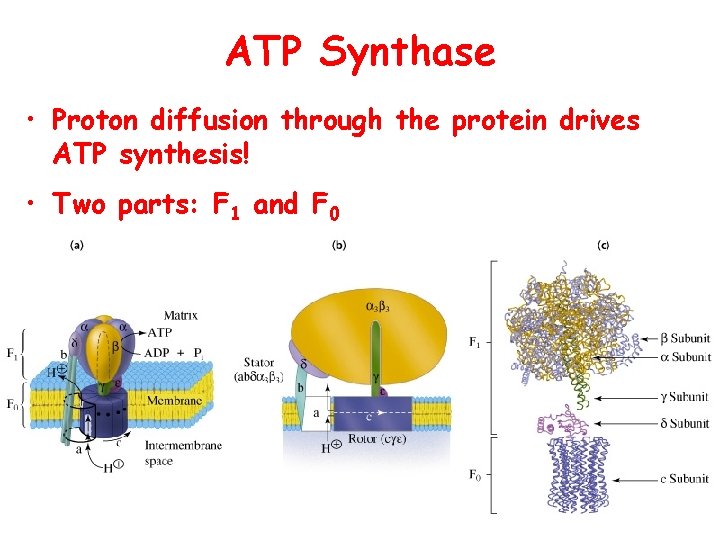
ATP Synthase • Proton diffusion through the protein drives ATP synthesis! • Two parts: F 1 and F 0

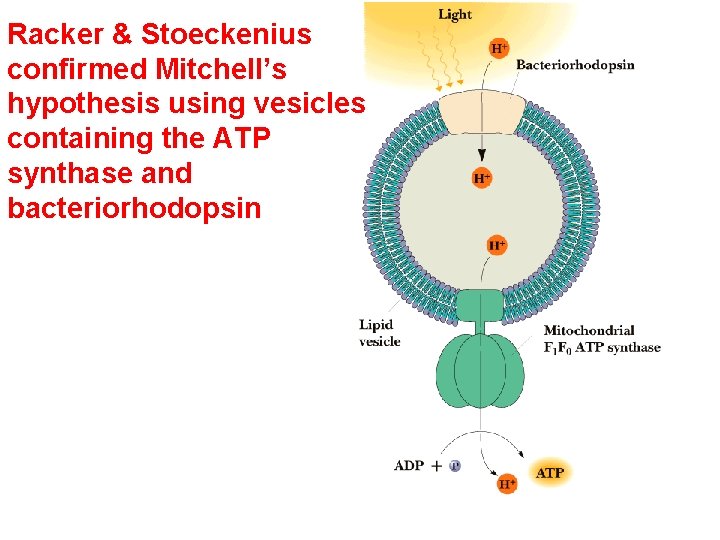
Racker & Stoeckenius confirmed Mitchell’s hypothesis using vesicles containing the ATP synthase and bacteriorhodopsin

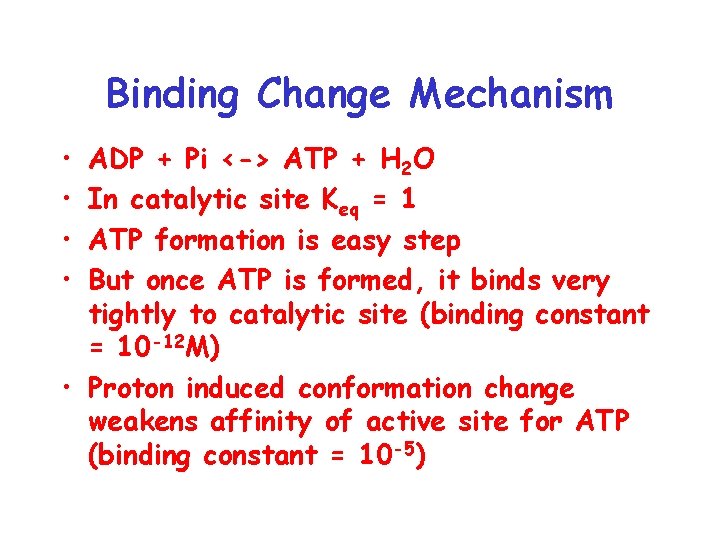
Binding Change Mechanism • • ADP + Pi <-> ATP + H 2 O In catalytic site Keq = 1 ATP formation is easy step But once ATP is formed, it binds very tightly to catalytic site (binding constant = 10 -12 M) • Proton induced conformation change weakens affinity of active site for ATP (binding constant = 10 -5)

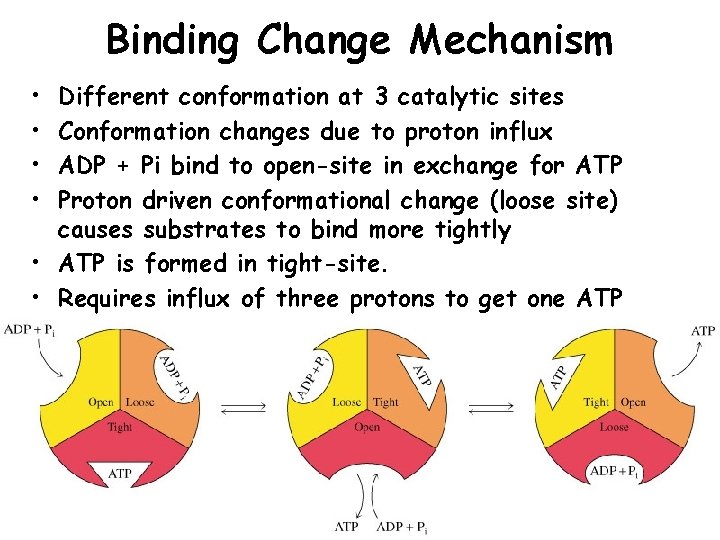
Binding Change Mechanism • • Different conformation at 3 catalytic sites Conformation changes due to proton influx ADP + Pi bind to open-site in exchange for ATP Proton driven conformational change (loose site) causes substrates to bind more tightly • ATP is formed in tight-site. • Requires influx of three protons to get one ATP

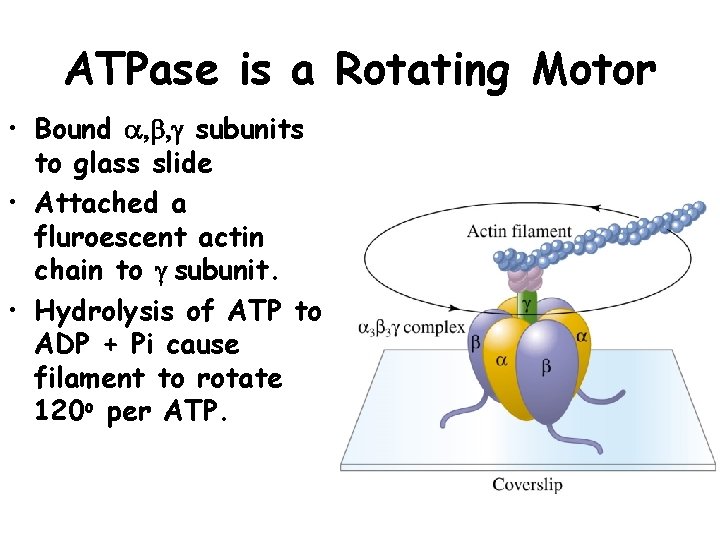
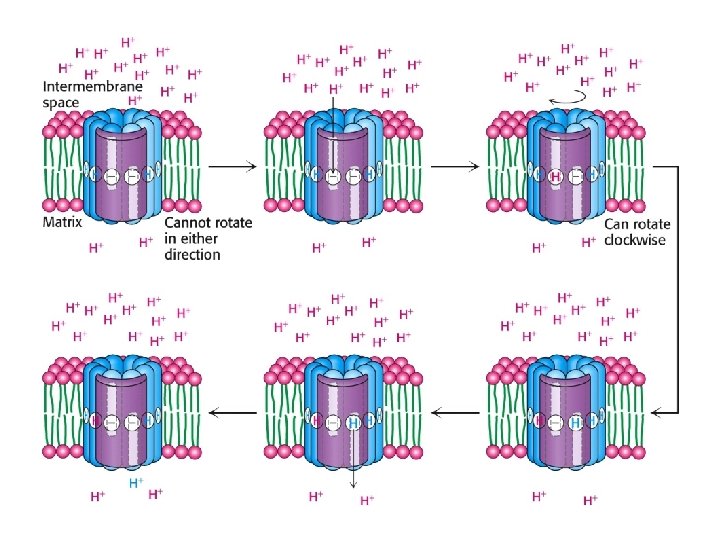
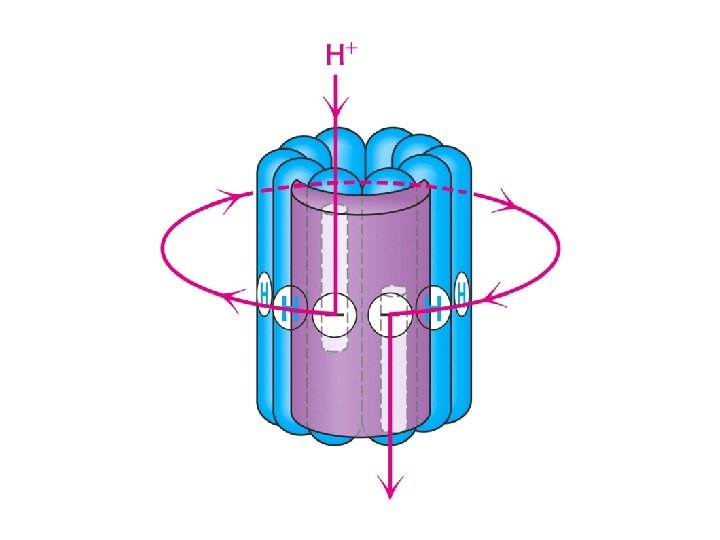
ATPase is a Rotating Motor • Bound a, b, g subunits to glass slide • Attached a fluroescent actin chain to g subunit. • Hydrolysis of ATP to ADP + Pi cause filament to rotate 120 o per ATP.

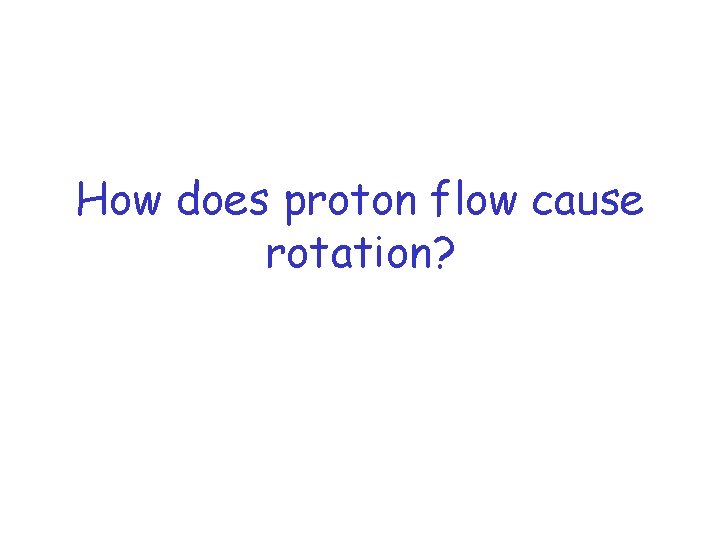
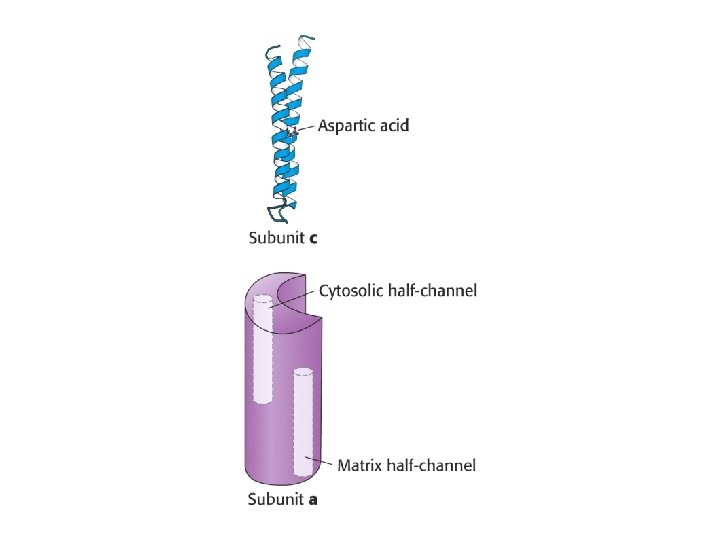
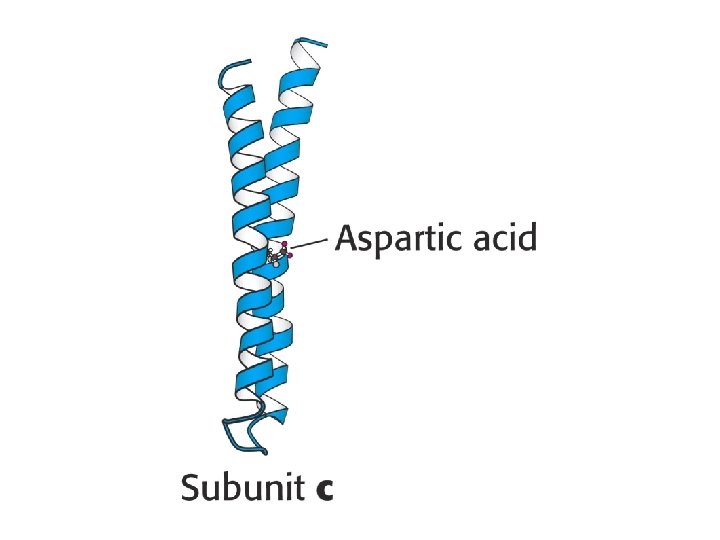
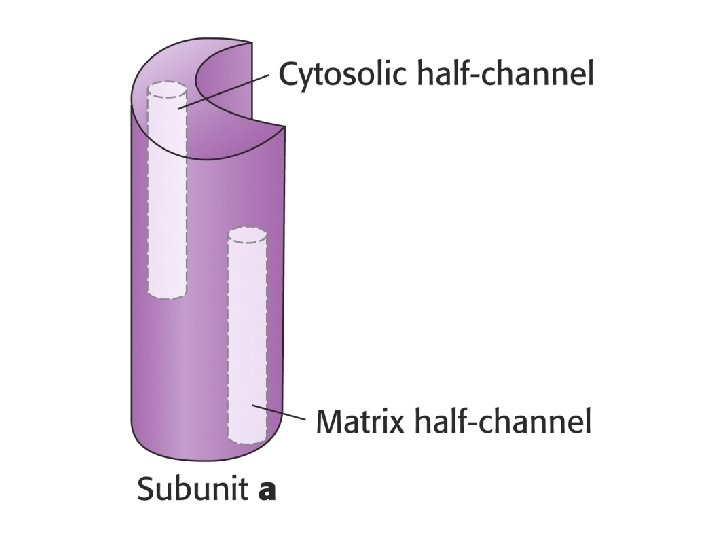
How does proton flow cause rotation?






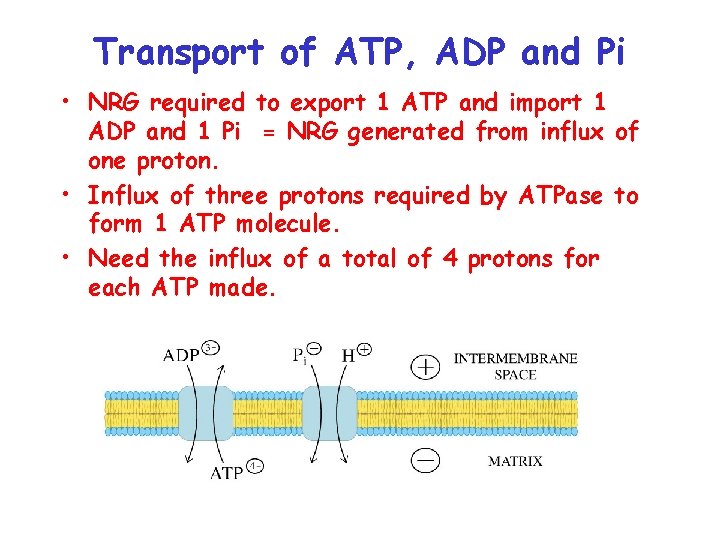
Active Transport of ATP, ADP and Pi Across Mitochondrial Inner Membrane • ATP is synthesized in the matrix • Need to export for use in other cell compartments • ADP and Pi must be imported into the matrix from the cytosol so more ATP can be made. • Require the use of transporters

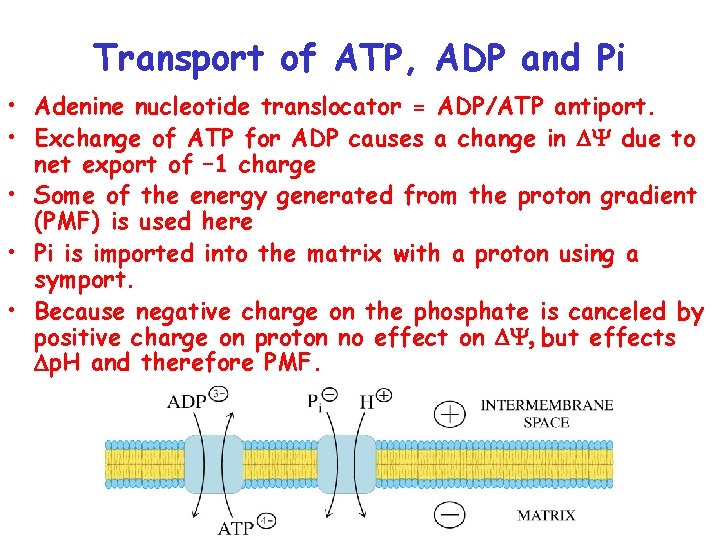
Transport of ATP, ADP and Pi • Adenine nucleotide translocator = ADP/ATP antiport. • Exchange of ATP for ADP causes a change in Y due to net export of – 1 charge • Some of the energy generated from the proton gradient (PMF) is used here • Pi is imported into the matrix with a proton using a symport. • Because negative charge on the phosphate is canceled by positive charge on proton no effect on Y, but effects p. H and therefore PMF.

Transport of ATP, ADP and Pi • NRG required to export 1 ATP and import 1 ADP and 1 Pi = NRG generated from influx of one proton. • Influx of three protons required by ATPase to form 1 ATP molecule. • Need the influx of a total of 4 protons for each ATP made.

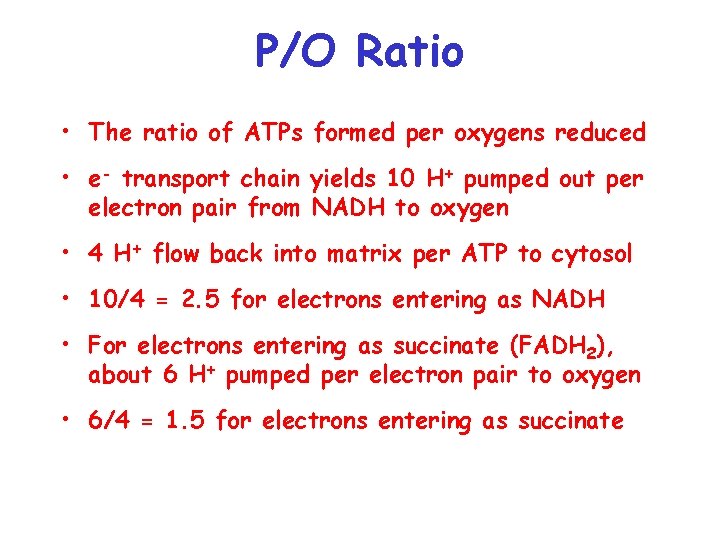
P/O Ratio • The ratio of ATPs formed per oxygens reduced • e- transport chain yields 10 H+ pumped out per electron pair from NADH to oxygen • 4 H+ flow back into matrix per ATP to cytosol • 10/4 = 2. 5 for electrons entering as NADH • For electrons entering as succinate (FADH 2), about 6 H+ pumped per electron pair to oxygen • 6/4 = 1. 5 for electrons entering as succinate

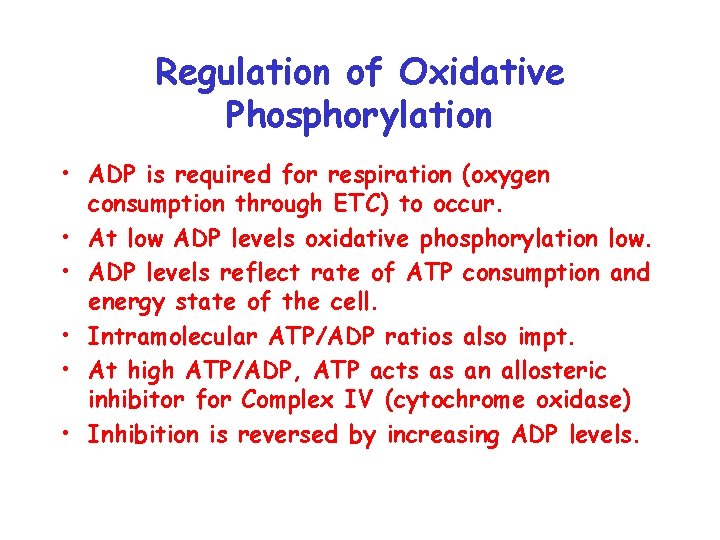
Regulation of Oxidative Phosphorylation • ADP is required for respiration (oxygen consumption through ETC) to occur. • At low ADP levels oxidative phosphorylation low. • ADP levels reflect rate of ATP consumption and energy state of the cell. • Intramolecular ATP/ADP ratios also impt. • At high ATP/ADP, ATP acts as an allosteric inhibitor for Complex IV (cytochrome oxidase) • Inhibition is reversed by increasing ADP levels.

Uncouplers • Uncouplers disrupt the tight coupling between electron transport and oxidative phosphorylation by dissipating the proton gradient • Uncouplers are hydrophobic molecules with a dissociable proton • They shuttle back and forth across the membrane, carrying protons to dissipate the gradient • w/o oxidative-phosphorylation energy lost as heat • Dinitrophenol once used as diet drug, people ran 107 o. F temperatures


Physiological Uncoupling • Uncoupling of ETC and Ox-phos occurs in animals as a means to produce heat = nonshivering thermogenesis. • Impt. In hibernating mammals, neborn animals and mammals adapted to cold • Occurs in brown adipose tissues (rich in mitochondria) • Uncoupling protein (UCP) = channel to allow influx of protons to matrix (dissipates proton gradient)


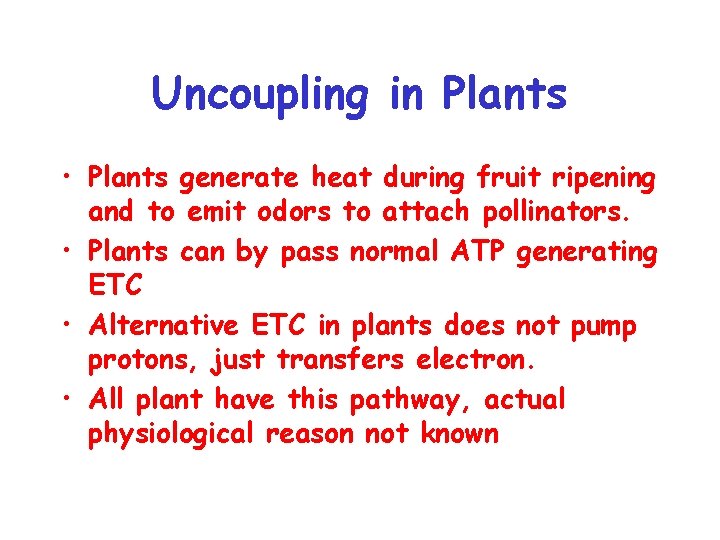
Uncoupling in Plants • Plants generate heat during fruit ripening and to emit odors to attach pollinators. • Plants can by pass normal ATP generating ETC • Alternative ETC in plants does not pump protons, just transfers electron. • All plant have this pathway, actual physiological reason not known
 Chemiosmotic theory
Chemiosmotic theory Chemiosmotic hypothesis explains
Chemiosmotic hypothesis explains Chemiosmotic hypothesis explains
Chemiosmotic hypothesis explains Energy
Energy Electrons carried in nadh diagram
Electrons carried in nadh diagram Unpaired electrons in electron configuration
Unpaired electrons in electron configuration Dot symbol text
Dot symbol text Electrons configurations
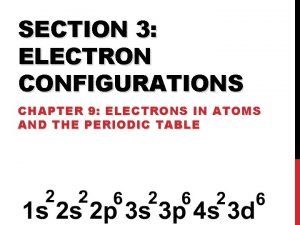
Electrons configurations Electron configuration for 5 electrons
Electron configuration for 5 electrons Oxydoreduction
Oxydoreduction Electrons in atoms section 2 quantum theory and the atom
Electrons in atoms section 2 quantum theory and the atom Electrons in atoms section 2 quantum theory and the atom
Electrons in atoms section 2 quantum theory and the atom Electron transport chain campbell
Electron transport chain campbell Energetics of oxidative phosphorylation
Energetics of oxidative phosphorylation Tca回路
Tca回路 Vertical
Vertical Uncouplers
Uncouplers Electron transport chain cellular respiration
Electron transport chain cellular respiration Where does the krebs cycle take place?
Where does the krebs cycle take place? Catabolism
Catabolism Electron transport chain cellular respiration
Electron transport chain cellular respiration Aerbobic
Aerbobic Electron transport chain
Electron transport chain Phosphorelation
Phosphorelation Chemiosmosis steps
Chemiosmosis steps Significance of electron transport chain
Significance of electron transport chain Photorepsiration
Photorepsiration Labelled
Labelled Mechanism of electron transport chain ppt
Mechanism of electron transport chain ppt Tca cycle animation
Tca cycle animation Citric acid cycle and electron transport chain
Citric acid cycle and electron transport chain Does chemiosmosis cause cramps
Does chemiosmosis cause cramps Returns outwards
Returns outwards The things they carried speaking of courage questions
The things they carried speaking of courage questions Why did azar kill the puppy
Why did azar kill the puppy Tttc quotes
Tttc quotes The things they carried theme
The things they carried theme Team work poem
Team work poem Syntax in the things they carried
Syntax in the things they carried The things they carried pov
The things they carried pov Balance carried down
Balance carried down Jacob moves to egypt
Jacob moves to egypt The secrets carried
The secrets carried Uses of trial balance
Uses of trial balance Fire hose appliances
Fire hose appliances The things they carried lessons
The things they carried lessons Mitchell sanders the things they carried
Mitchell sanders the things they carried The things they carried plot summary
The things they carried plot summary Hot working of metal is
Hot working of metal is