Electron Transport and Oxidative Phosphorylation 1 Metabolism Overview

Electron Transport and Oxidative Phosphorylation 1
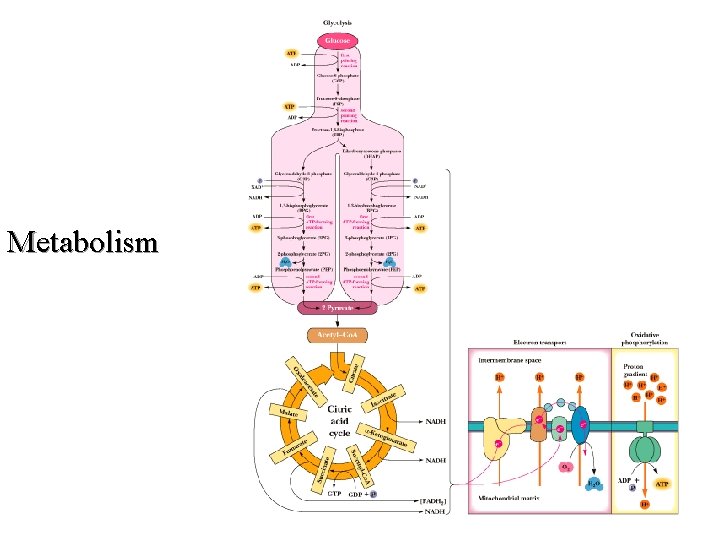
Metabolism
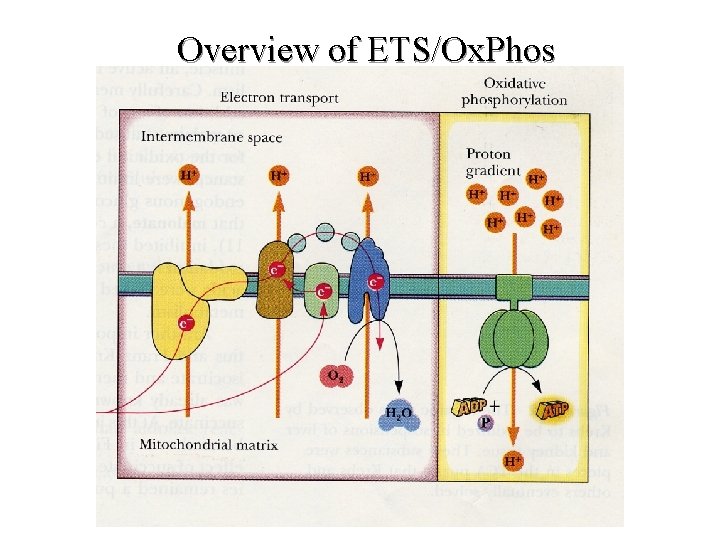
Overview of ETS/Ox. Phos
Overview of ETS/Ox. Phos, Fig. 18. 2
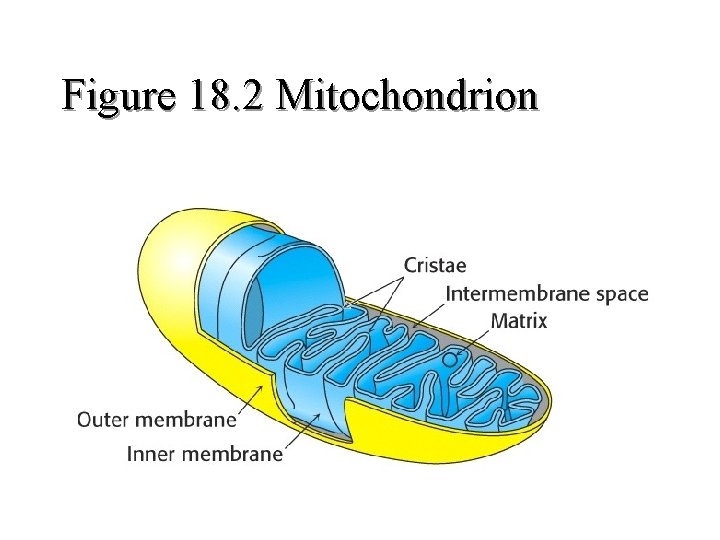
Figure 18. 2 Mitochondrion

Objectives Prove that the ATP yield from the metabolism of 1. 0 mole of glucose oxidized via glycolysis and the TCA cycle pathways produces 30/32 moles of ATP. A. 6
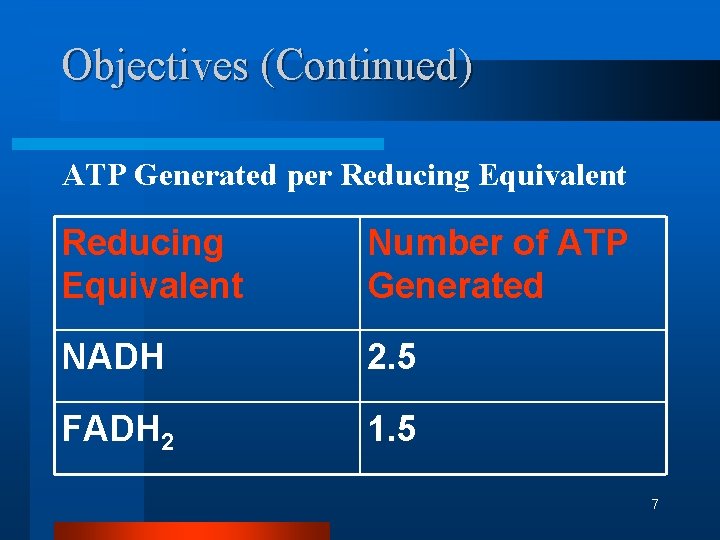
Objectives (Continued) ATP Generated per Reducing Equivalent Number of ATP Generated NADH 2. 5 FADH 2 1. 5 7

Objectives (Continued) l B. Define • 1. • 2. • 3. • 4. • 5. An oxidation reaction A reduction reaction Oxidative phosphorylation An uncoupler Redox potential 8

Objectives (Continued) l C. List: 1. 2. 3. 4. 5. 6. The components of the electron transfer chain in sequential order. The sites where ATP is produced in the electron transfer chain. The inhibitors of electron transfer. The uncouplers of oxidative Phosphorylation. The inhibitors of oxidative Phosphorylation. The stimulators of electron transport 9

Objectives (Continued) • D. Compare/Contrast the glycerol 3 -phosphate shuttle with the malate -Aspartate shuttle with respect to: • 1. • 2. • 3. Participating electron carriers Transport of reducing equivalents Tissue specificity 10

Objectives (Continued) l E. Discuss Mitchell's Chemiosmotic Theory of oxidative phosphorylation • 1. • 2. • 3. Diagram the system. Point out its features. Explain how the system works. 11

Objectives (Continued) l F. Discuss the function of electron transport and oxidative phosphorylation 12
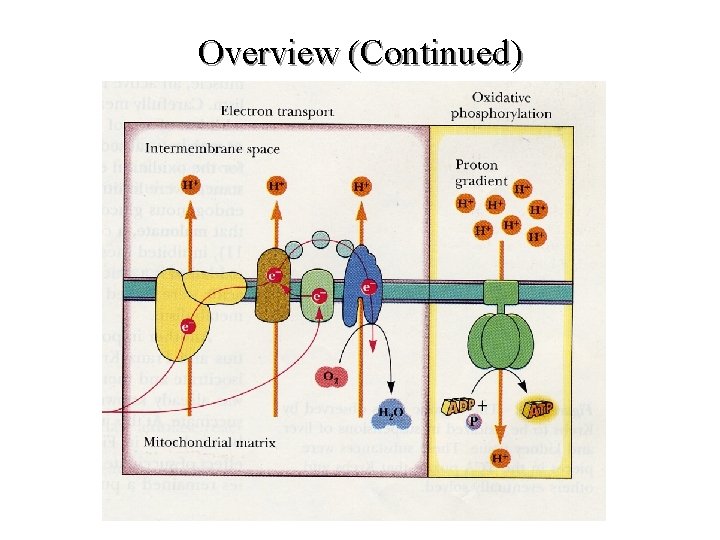
Overview (Continued)
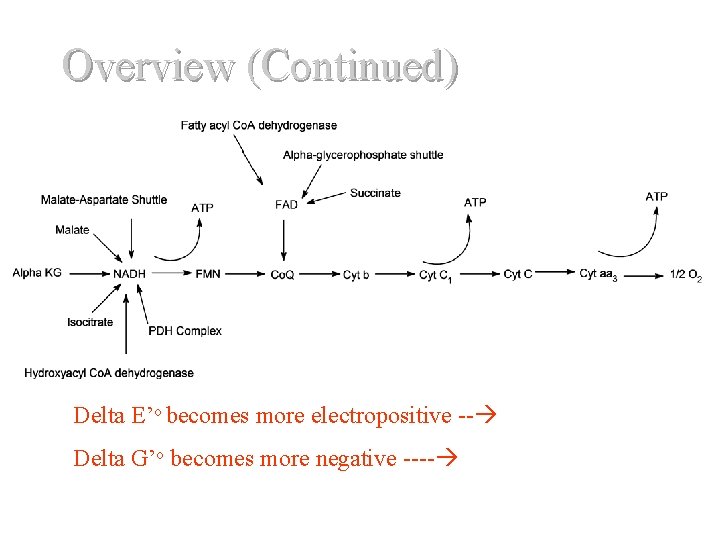
Overview (Continued) D Delta E’o becomes more electropositive -- Delta G’o becomes more negative ---- Del
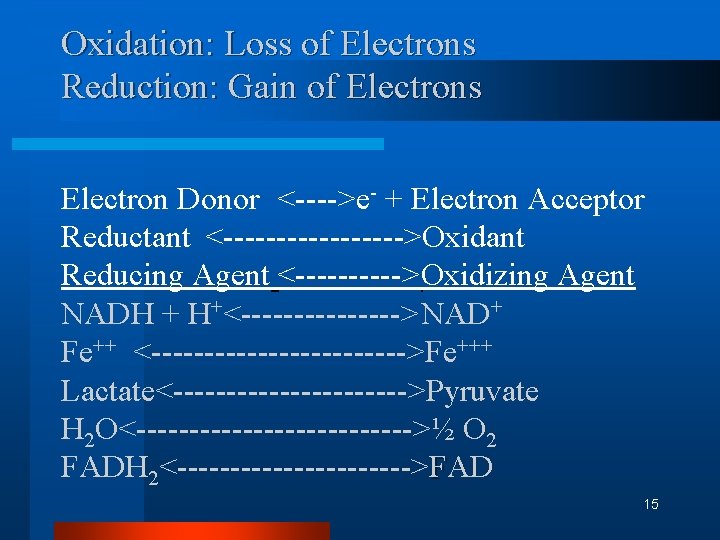
Oxidation: Loss of Electrons Reduction: Gain of Electrons Electron Donor <---->e- + Electron Acceptor Reductant <--------->Oxidant Reducing Agent <----->Oxidizing Agent NADH + H+<-------->NAD+ Fe++ <------------>Fe+++ Lactate<----------->Pyruvate H 2 O<------------->½ O 2 FADH 2<----------->FAD 15

I. Definitions l Oxidizing agent is the electron acceptor l Reducing agent is the electron donor. 16

Definitions (Continued K 2) I. C. Half-reaction or redox couple is a way to show the tendency of an element or compound to gain or lose electrons. The convention is electron acceptor + ne <-----> electron donor 17

I. Definitions (Continued K 2) D. This tendency to gain or lose electrons is quantified by the standard redox potential (E 0'). 1. the more positive the standard redox potential, the stronger the attraction for electrons. 2. the most positive couple always accepts electrons from the least positive couple. 18
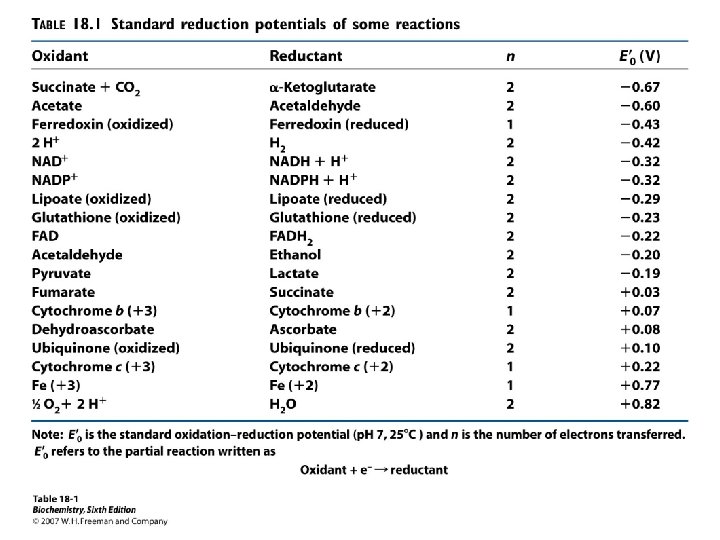

I. Definitions (Continued K 3) E. When the concentrations of electron donor and electron acceptor of a particular redox couple are equal, then ΔE 0 N = E 0 N(electron acceptor) - E 0 N(electron donor) 20

I. Definitions (Continued K 2) F. If the concentrations of the electron donor and electron acceptor are not equal then the Nernst Equation is used to calculate the reduction potential for the half-reaction. Once the "corrected" E' is calculated the overall E' for the reaction is calculated using the equation on previous slide. 21
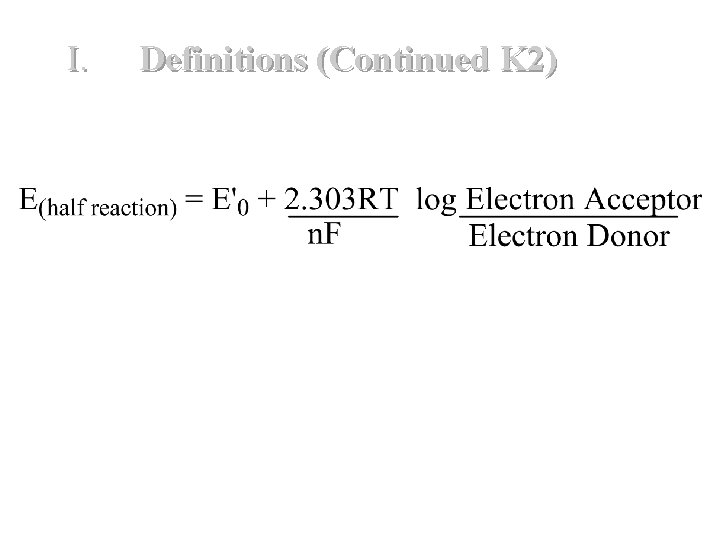
I. Definitions (Continued K 2) where E & 0 = standard redox potential for half reaction in volts R = gas constant = 8. 31 Joule/deg K/mole T = Absolute Temperature in 0 K degrees n = number of electrons transferred
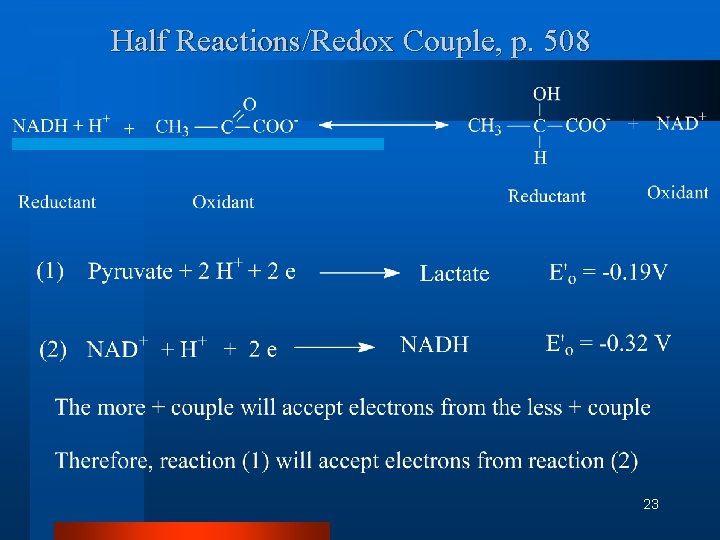
Half Reactions/Redox Couple, p. 508 23
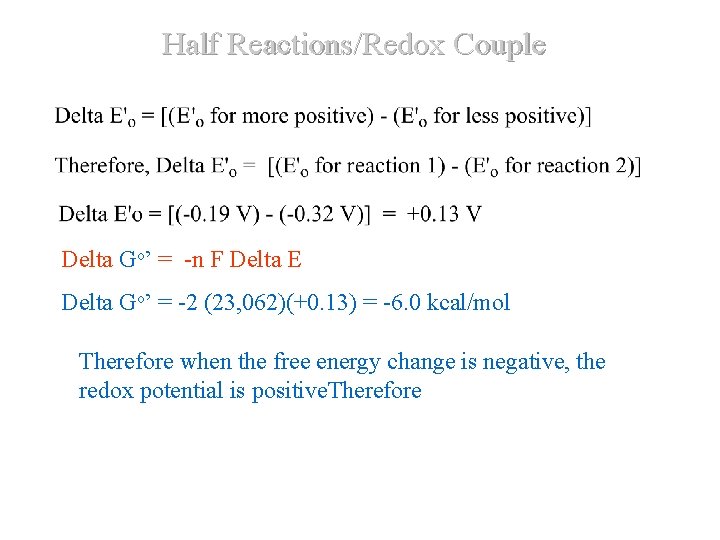
Half Reactions/Redox Couple Delta Go’ = -n 0 F Delta E ΔG ' = -n F E 0' Delta Go’ = -2 (23, 062)(+0. 13) = -6. 0 kcal/mol. DDet. D Therefore when the free energy change is negative, the redox potential is positive. Therefore
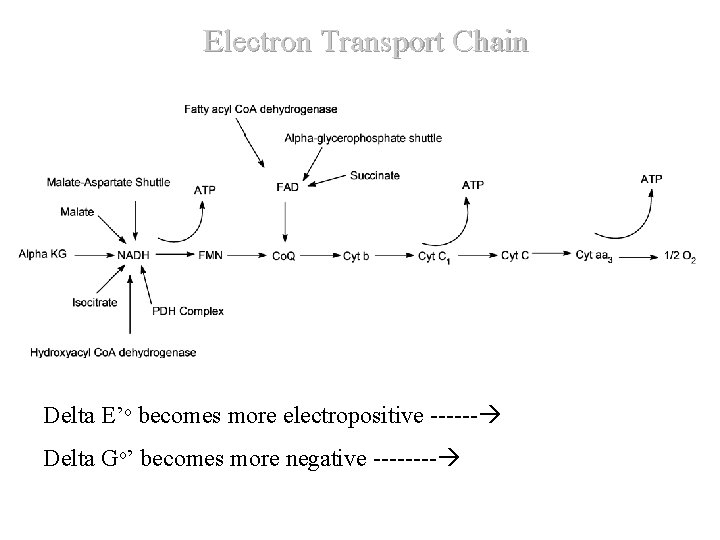
Electron Transport Chain Delta E’o becomes more electropositive ------ Delta Go’ becomes more negative ---- D
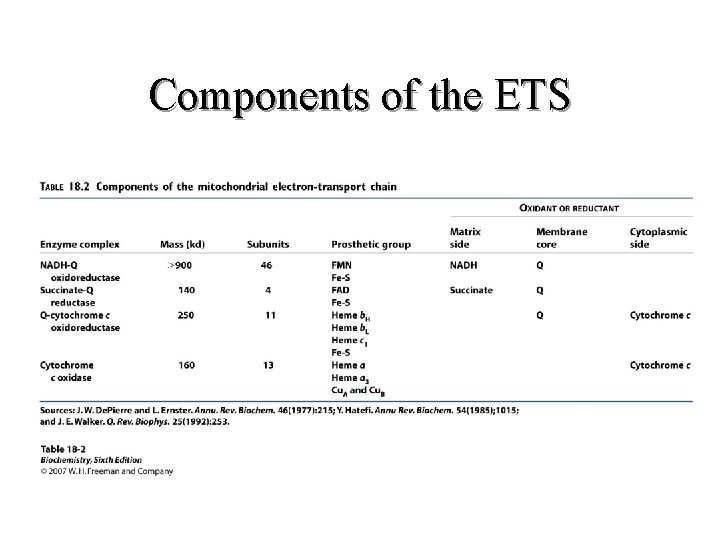
Components of the ETS
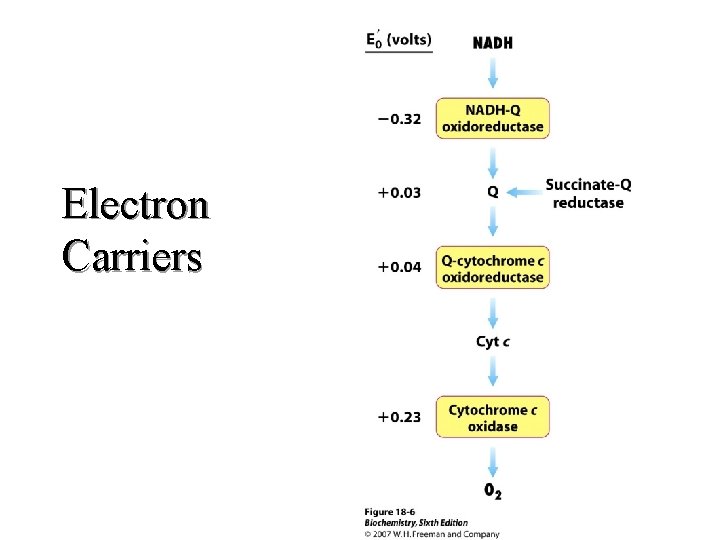
Electron Carriers
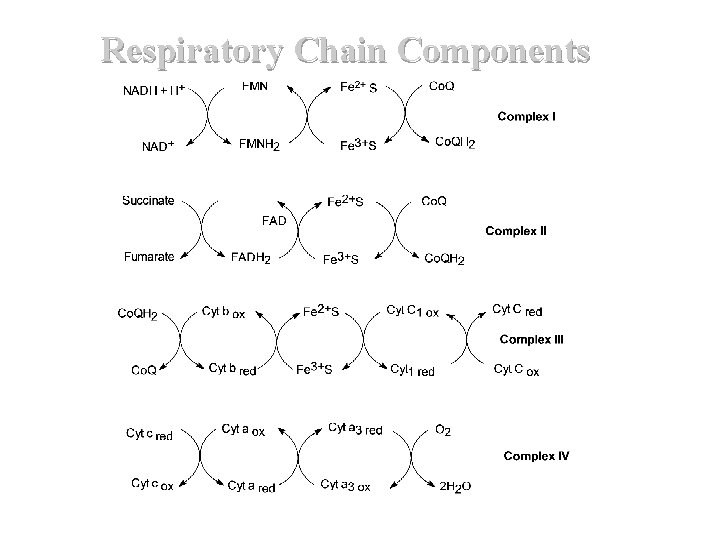
Respiratory Chain Components

II. Respiratory Chain Components (K 3) 1. Coenzymes a. NAD(H) (1) carries one proton and two electrons (hydride) (b) carbon 4 of the pyrimidine ring is the "active" site (c) unbound 29
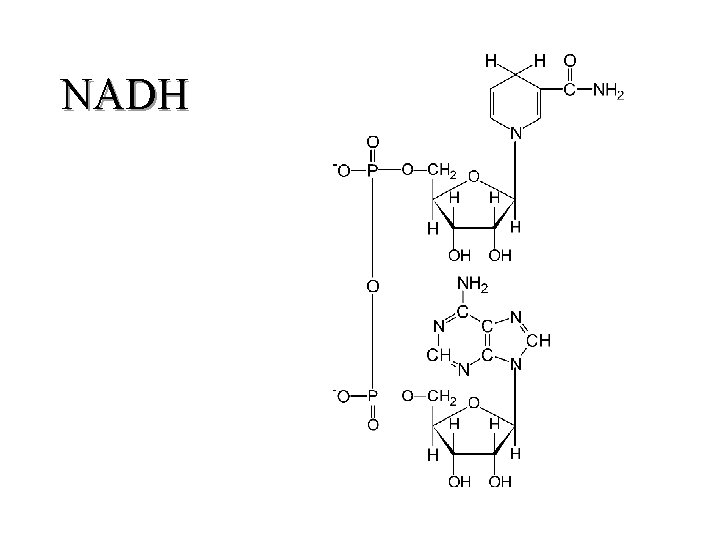
NADH
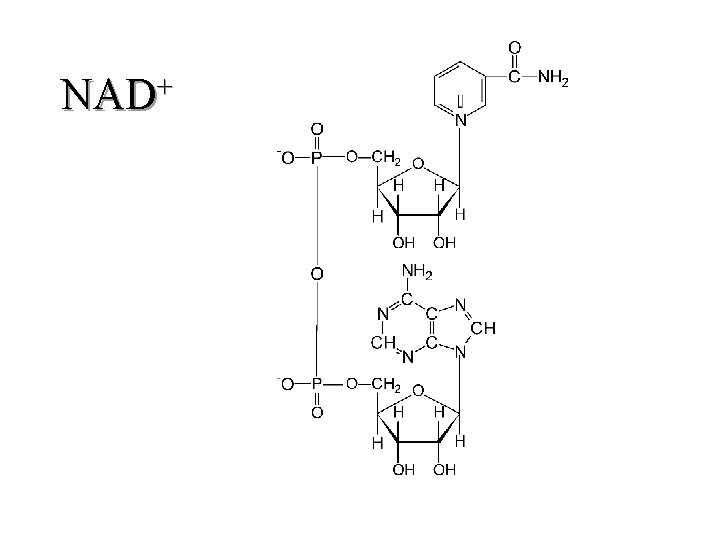
+ NAD
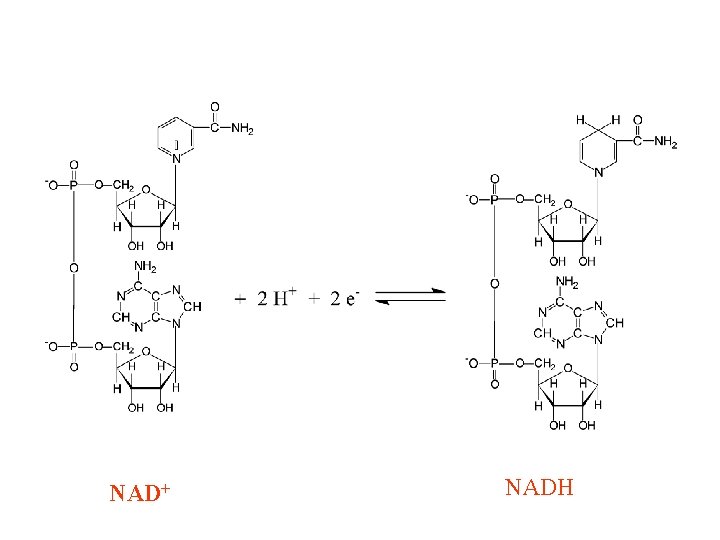
+ NADH
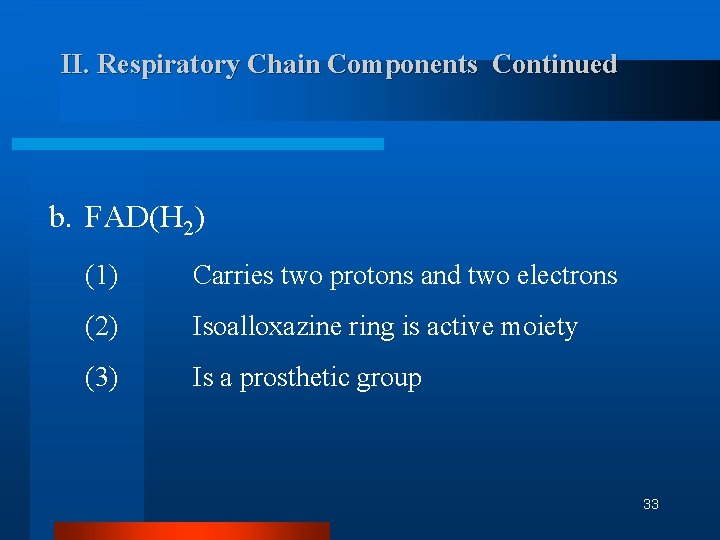
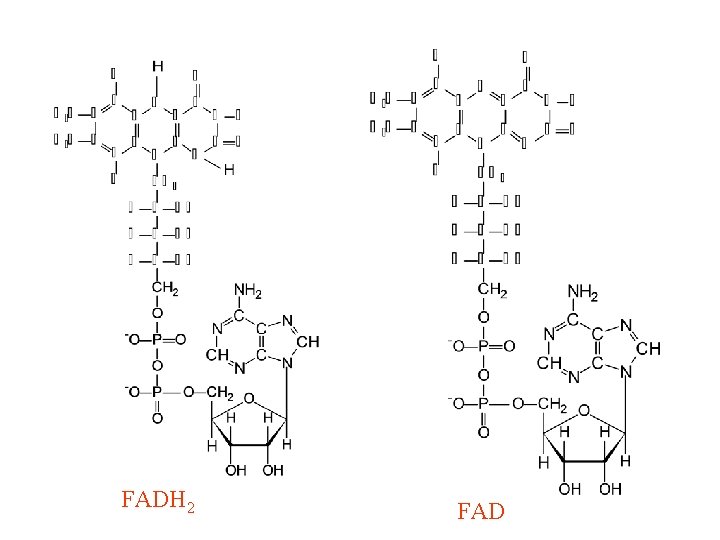
II. Respiratory Chain Components Continued b. FAD(H 2) (1) Carries two protons and two electrons (2) Isoalloxazine ring is active moiety (3) Is a prosthetic group 33
II. Respiratory Chain Components Continued FAD(H 2) Figure 14. 15 in text And on Blackboard 34
FADH 2 FAD
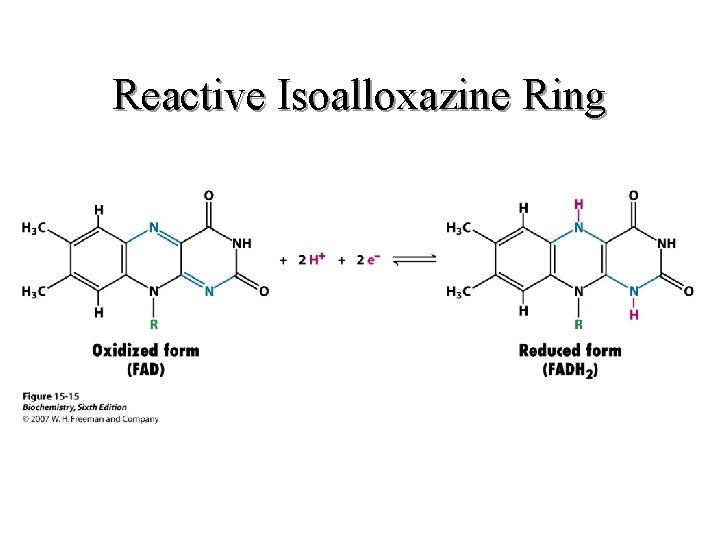
Reactive Isoalloxazine Ring
II. Respiratory Chain Components Continued C. FMN(H 2) (1) Carries two protons and two electrons (2) Isoalloxazine ring is active moiety (3) Is a prosthetic group 37
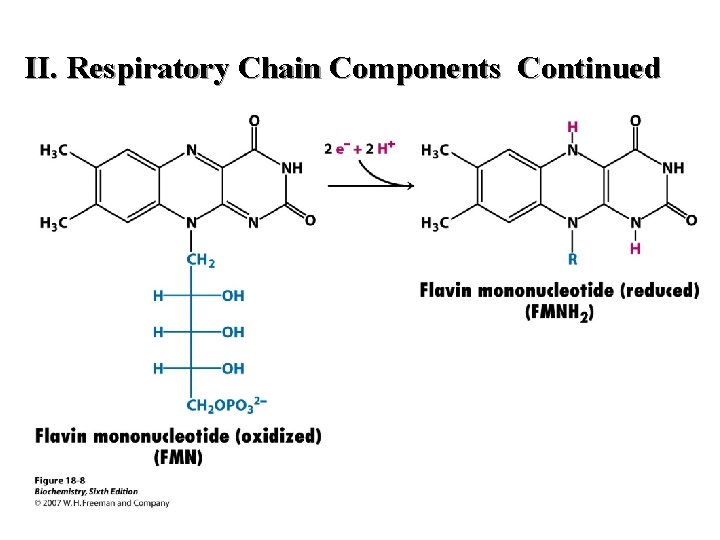
II. Respiratory Chain Components Continued

Iron-Sulfur Clusters (Fe++S l NADH-Co. Q oxidoreductase prosthetic Group l Receives electrons from FMNH 2 and transfers them to Co. Q l No protons are released l Non-heme iron proteins 39
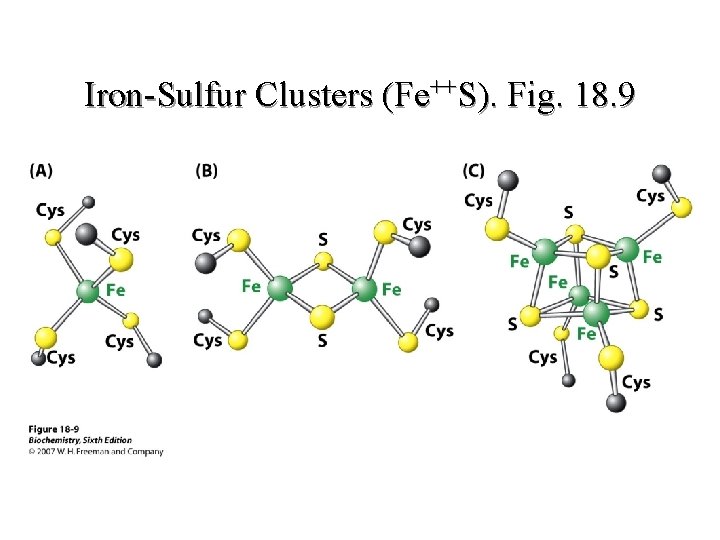
Iron-Sulfur Clusters (Fe++S). Fig. 18. 9

II. Respiratory Chain Components Continued D. Co. Q (not a coenzyme in the strict sense) or Ubiquinone (1) gains the equivalent of two electrons and two protons (2) lipid in quinone class of compounds; variable chain lenth (3) quinone structure is active site 41
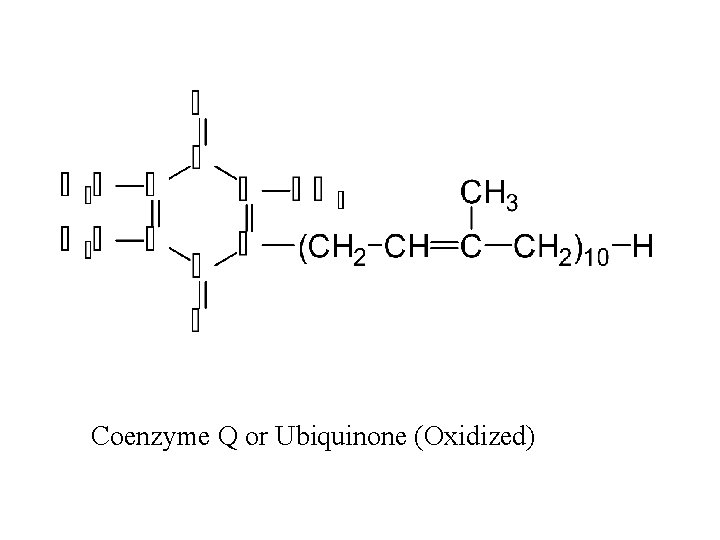
Coenzyme Q or Ubiquinone (Oxidized)
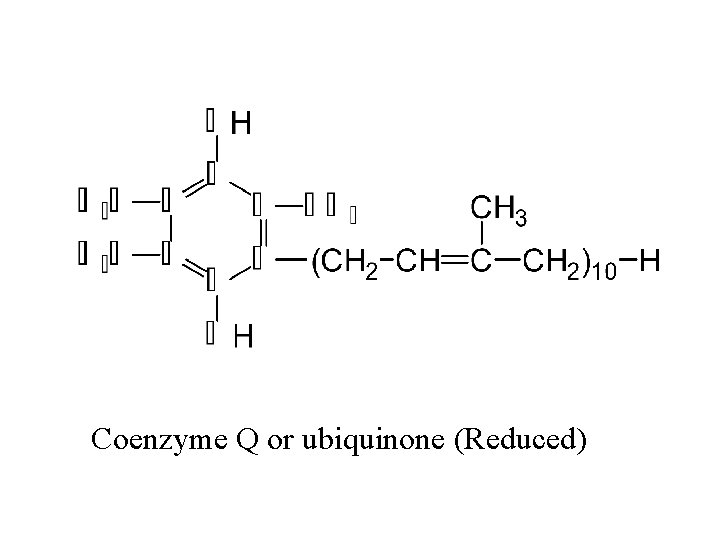
Coenzyme Q or ubiquinone (Reduced)
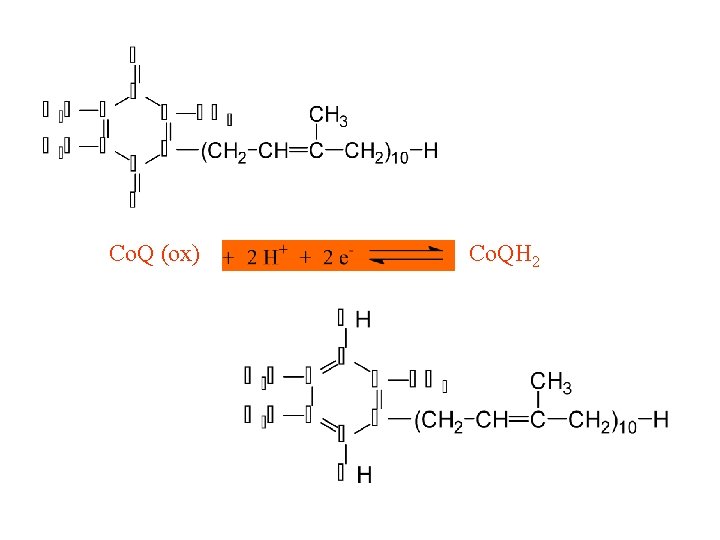
Co. Q (ox) Co. QH 2
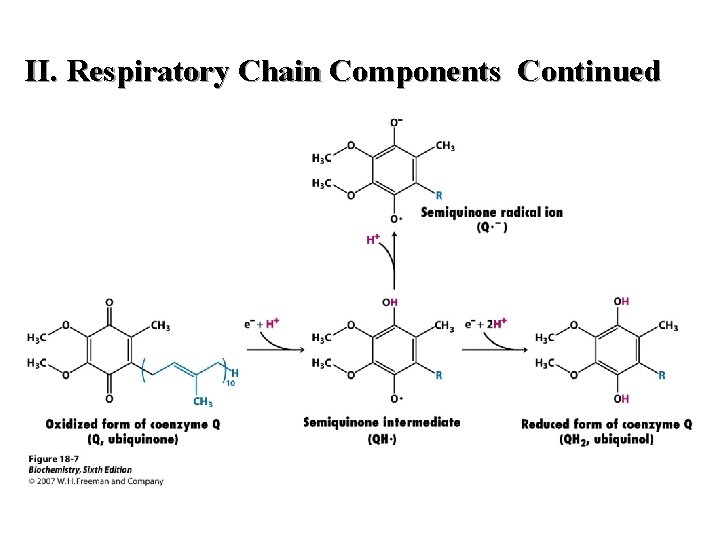
II. Respiratory Chain Components Continued
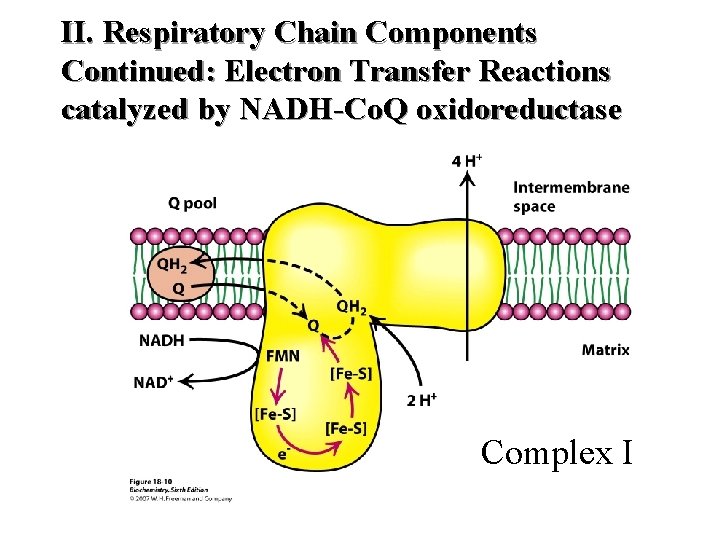
II. Respiratory Chain Components Continued: Electron Transfer Reactions catalyzed by NADH-Co. Q oxidoreductase Complex I
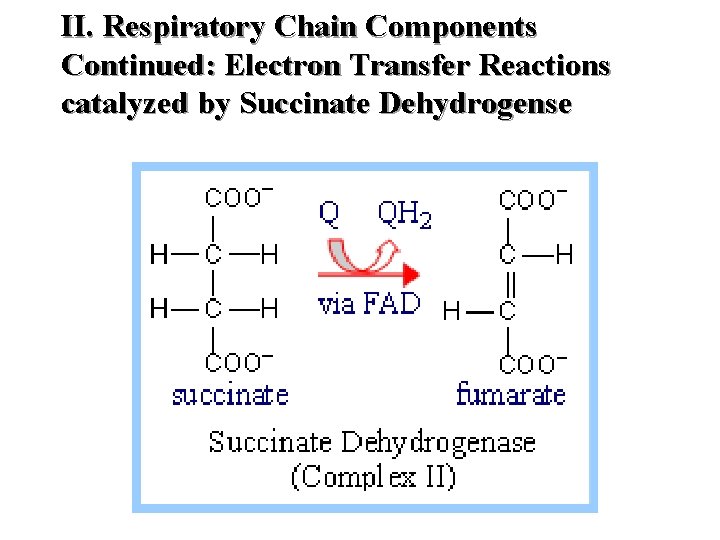
II. Respiratory Chain Components Continued: Electron Transfer Reactions catalyzed by Succinate Dehydrogense
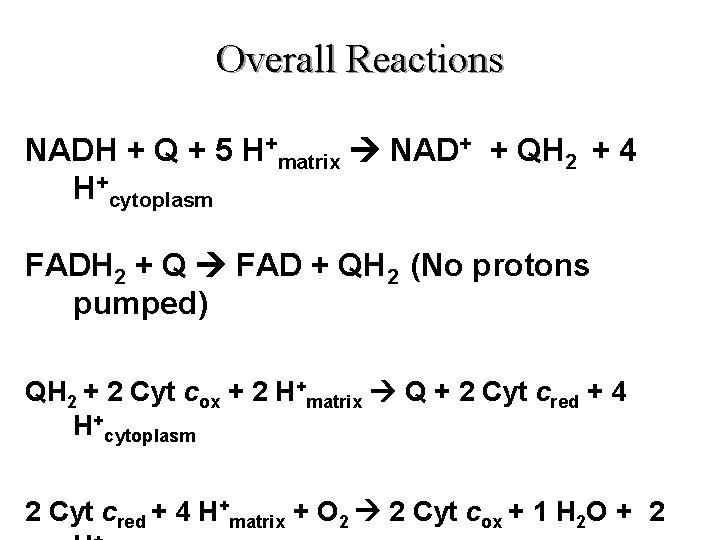
Overall Reactions NADH + Q + 5 H+matrix NAD+ + QH 2 + 4 H+cytoplasm FADH 2 + Q FAD + QH 2 (No protons pumped) QH 2 + 2 Cyt cox + 2 H+matrix Q + 2 Cyt cred + 4 H+cytoplasm 2 Cyt cred + 4 H+matrix + O 2 2 Cyt cox + 1 H 2 O + 2
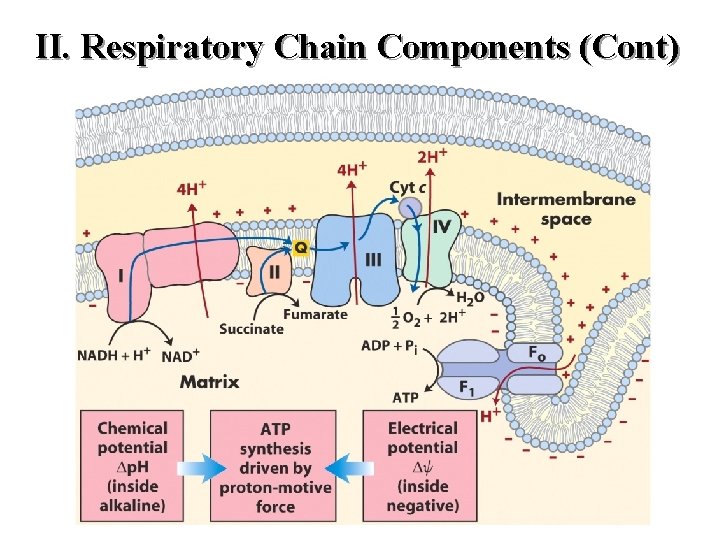
II. Respiratory Chain Components (Cont)
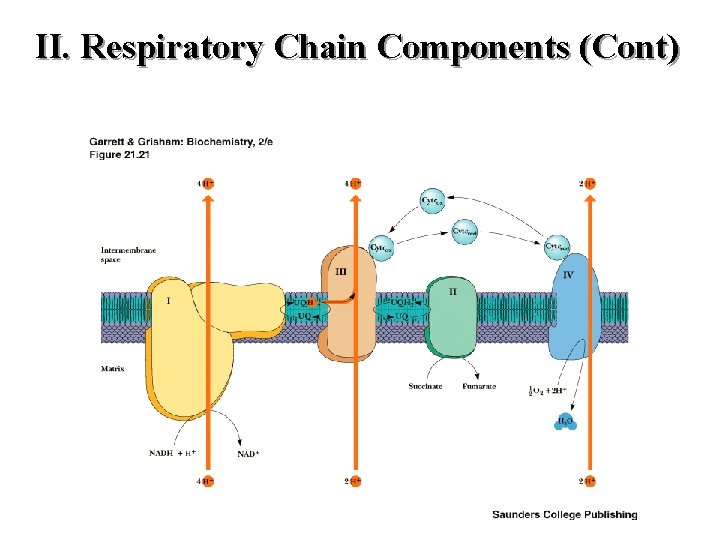
II. Respiratory Chain Components (Cont)
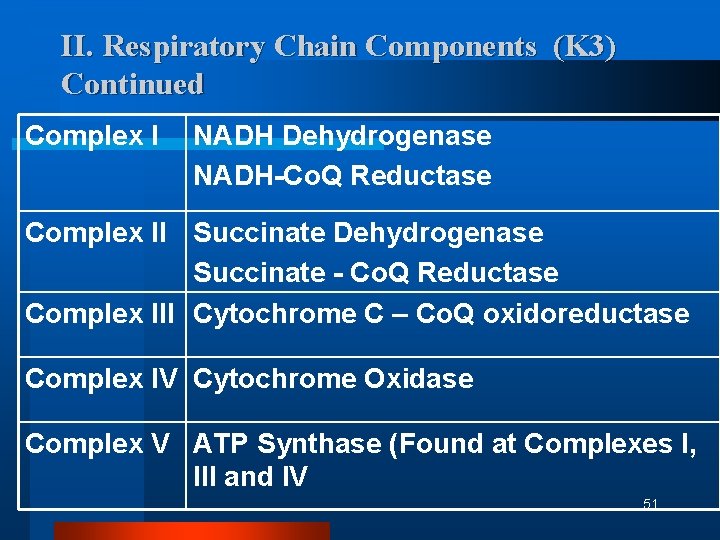
II. Respiratory Chain Components (K 3) Continued Complex I NADH Dehydrogenase NADH-Co. Q Reductase Complex II Succinate Dehydrogenase Succinate - Co. Q Reductase Complex III Cytochrome C – Co. Q oxidoreductase Complex IV Cytochrome Oxidase Complex V ATP Synthase (Found at Complexes I, III and IV 51
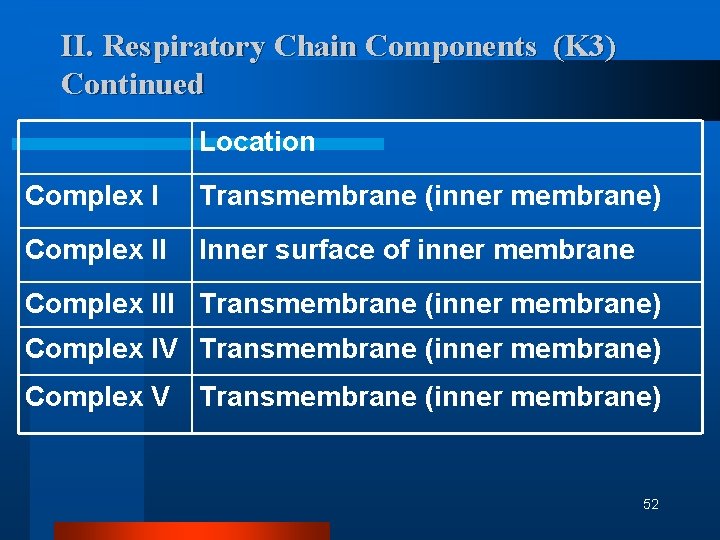
II. Respiratory Chain Components (K 3) Continued Location Complex I Transmembrane (inner membrane) Complex II Inner surface of inner membrane Complex III Transmembrane (inner membrane) Complex IV Transmembrane (inner membrane) Complex V Transmembrane (inner membrane) 52

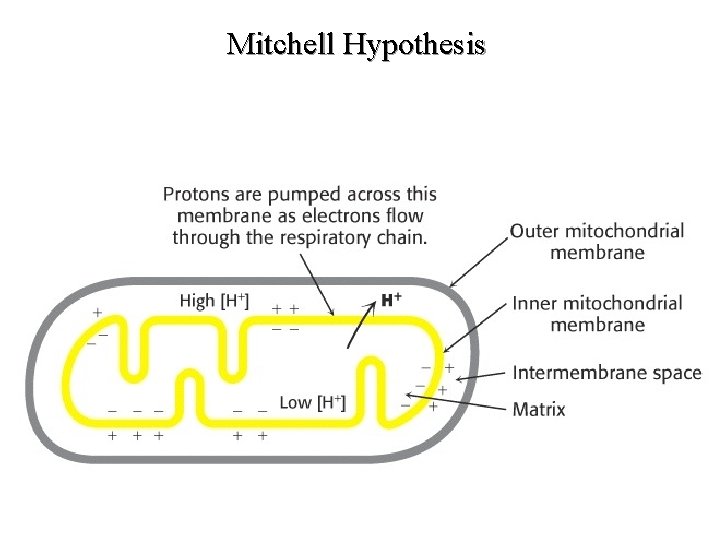
Mitchell Hypothesis (p. AA 18) The Mitchell Hypothesis or "Chemical Coupling Hypothesis" is the most widely accepted theoretical explanation of oxidative phosphorylation (non-substrate level ATP biosynthesis from ATP and Pi). Studies with 2, 4 -dinitrophenol and other uncoupling agents have made it quite clear that oxidative phosphorylation is obligatorily linked to electron transport. As a corollary, it has been proposed that the energy released as electrons pass from NADH to molecular oxygen is used to catalyze ATP biosynthesis. This model is based on the assumptions listed below. 53

l The mitochondrial inner membrane is impermeable to the free movement of protons (H+) and hydroxyl ions (-OH). l Therefore, the transport and exit of protons from the matrix and inner membrane, respectively, to the space and beyond must be an energy driven process.

l This energy is derived from the movement of electrons down the electron transport chain. While the electrons continue their passage, protons are separated away and discharged in a vectorial manner into the space. Complexes I, III and IV are theorized to act as pumps to facilitate the ejection of the charged protons into the space.
l As a consequence of the translocation of protons to the space, an electrochemical gradient (both charge and concentration) forms across the inner membrane. This results in the space becoming more electropositive accompanied by a drop in the p. H. The matrix by default becomes more electronegative with a concomitant increase in p. H.

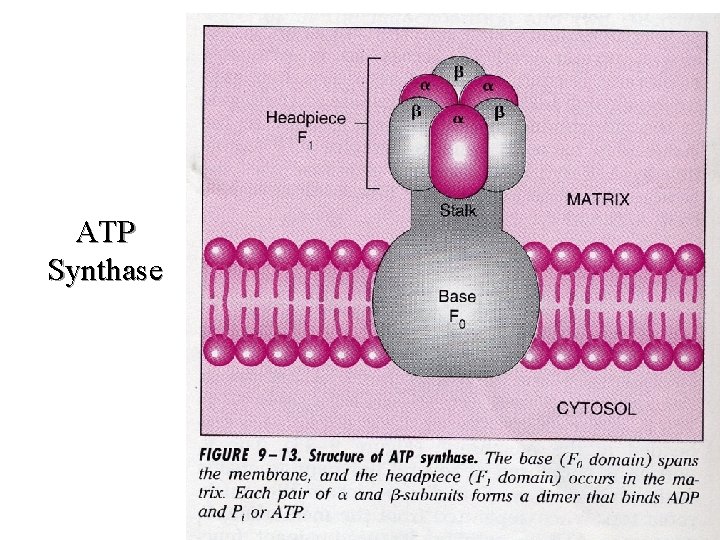
l The ATP synthase enzyme found in special pores in the inner membrane catalyzes the formation of ATP. This occurs as a function of the dissipation of energy as protons pass down a concentration gradient back to the matrix. The actual mechanism by which energy dissipation leads to increased catalytic activity of the ATP synthase remains to be determined.
Mitchell Hypothesis
ATP Synthase
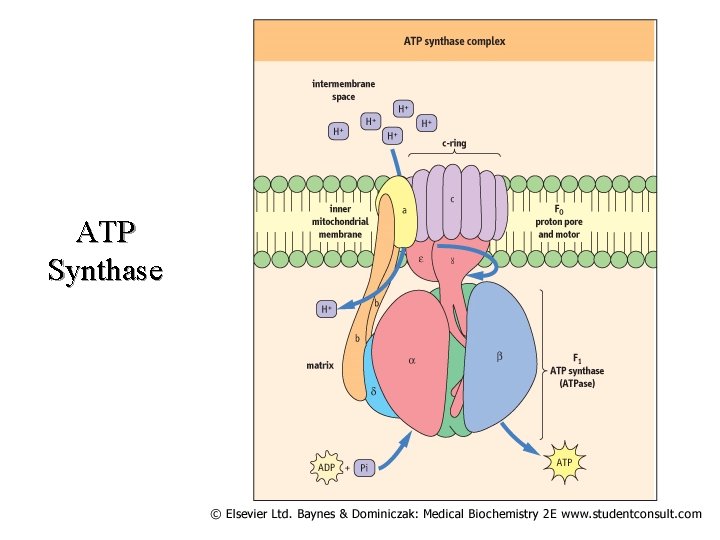
ATP Synthase

ATP Synthase
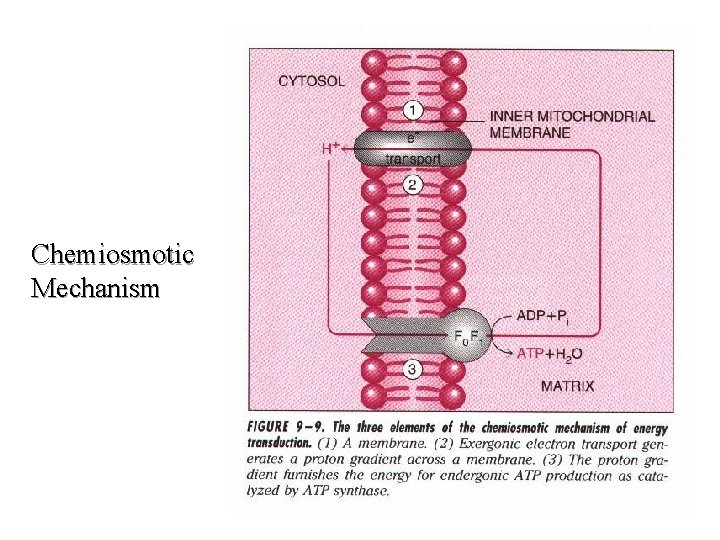
Chemiosmotic Mechanism
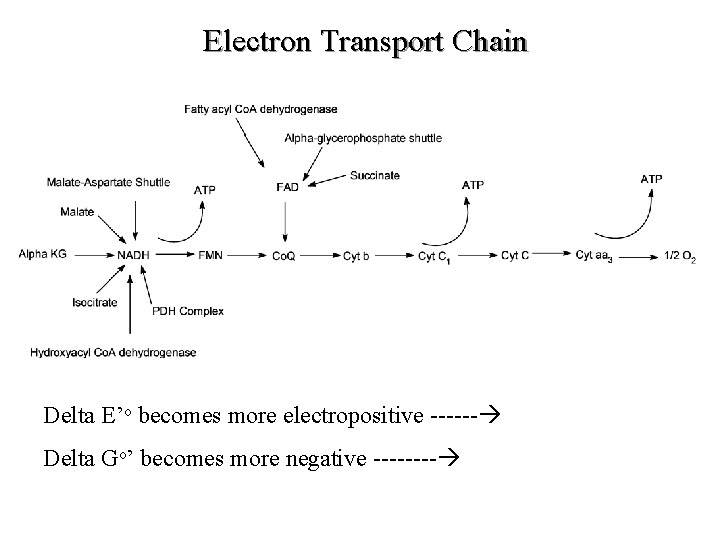
Electron Transport Chain Delta E’o becomes more electropositive ------ Delta Go’ becomes more negative ---- D
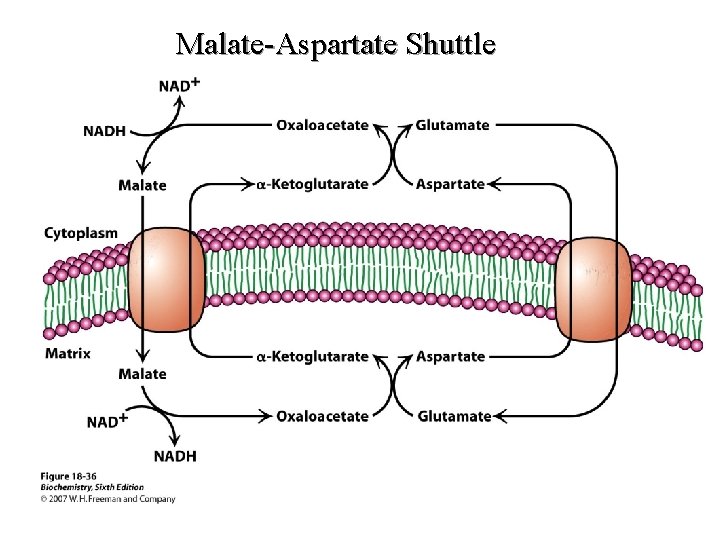
Malate-Aspartate Shuttle
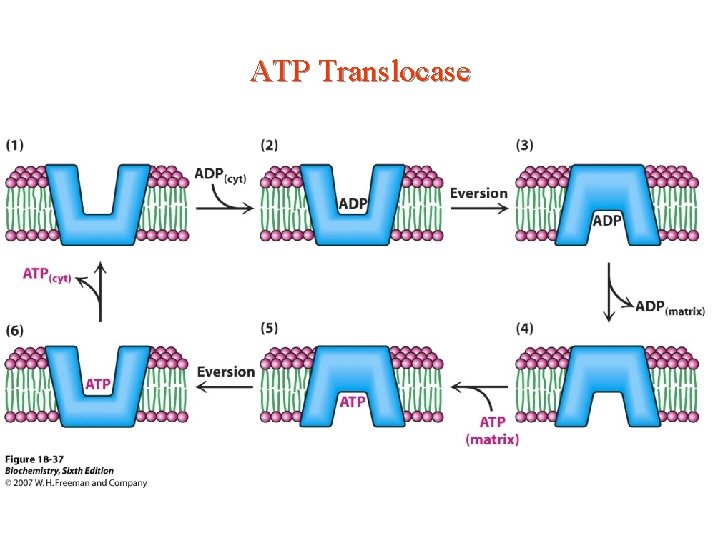
ATP Translocase
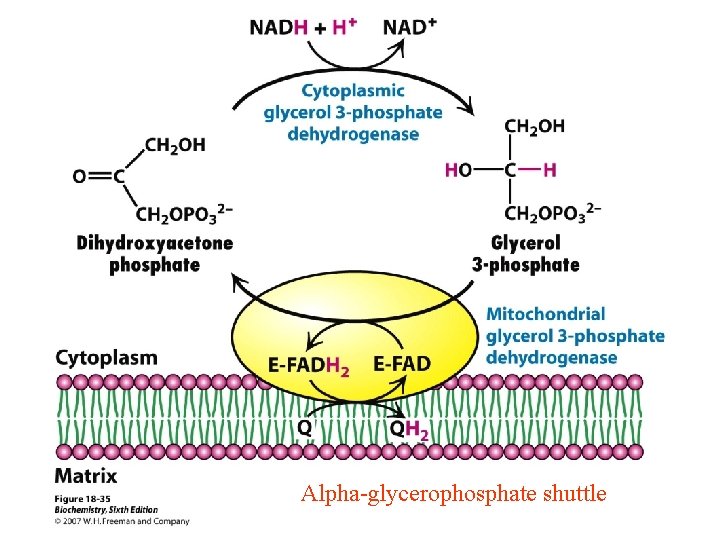
Alpha-Glycerol Phosphate Shuttle Alpha-glycerophosphate shuttle
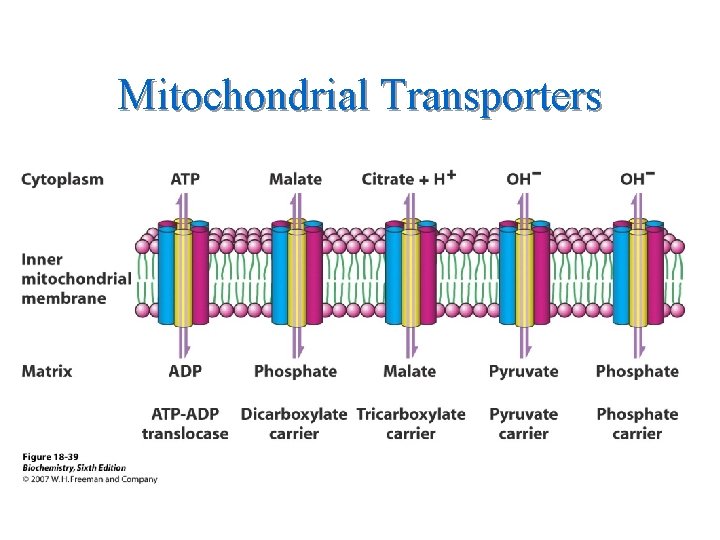
Mitochondrial Transporters
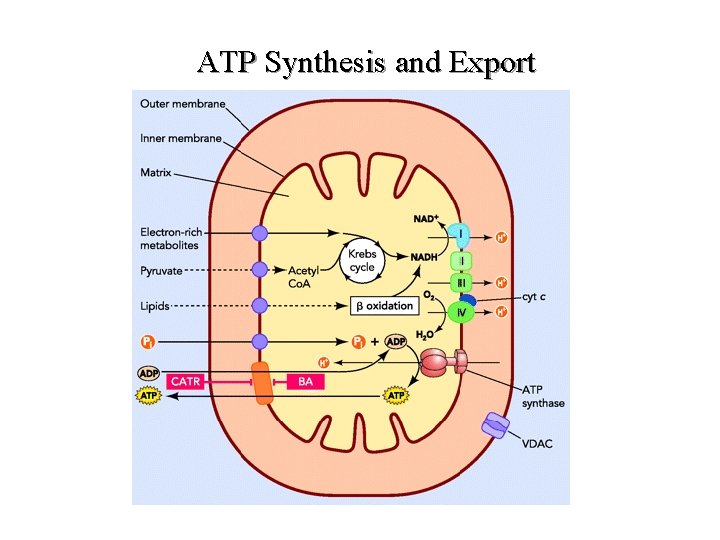
ATP Synthesis and Export
ATP Synthesis and Export l l l Reducing equivalents from electron-rich metabolites are oxidized by the respiratory chain. The resulting transmembrane proton gradient is used by the ATP synthase to produce ATP from ADP and phosphate. Phosphate ions are imported into - exchanger. Channelling of ADP, entering mitochondria, mitochondria thanks to a Pi/OH and ATP exported toward the cytosol is operated by the ADP/ATP carrier (orange box). Abreviations: BA, bongkrekic acid; CATR, carboxyatractyloside; IM, inner mitochondrial membrane; OM, outer mitochondrial membrane; VDAC, voltage-dependent anion channel.
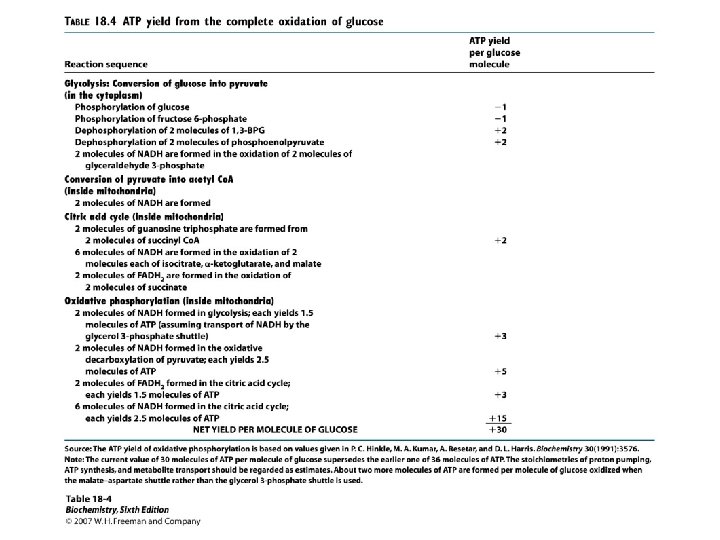
ATP Yield
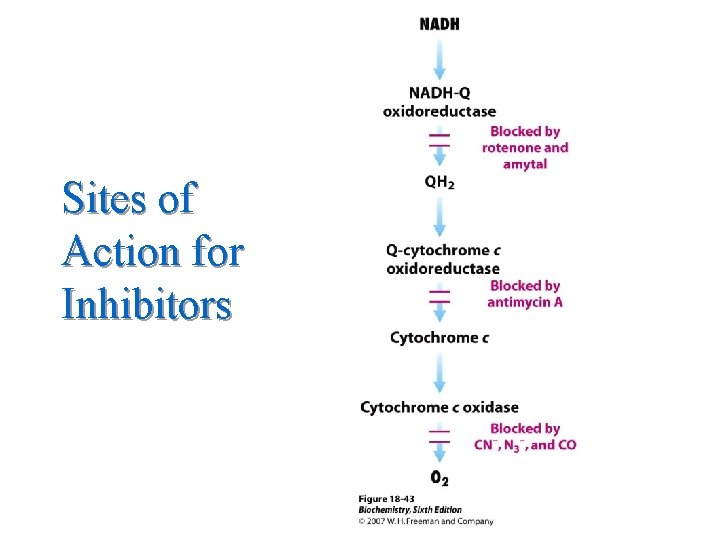
Sites of Action for Inhibitors
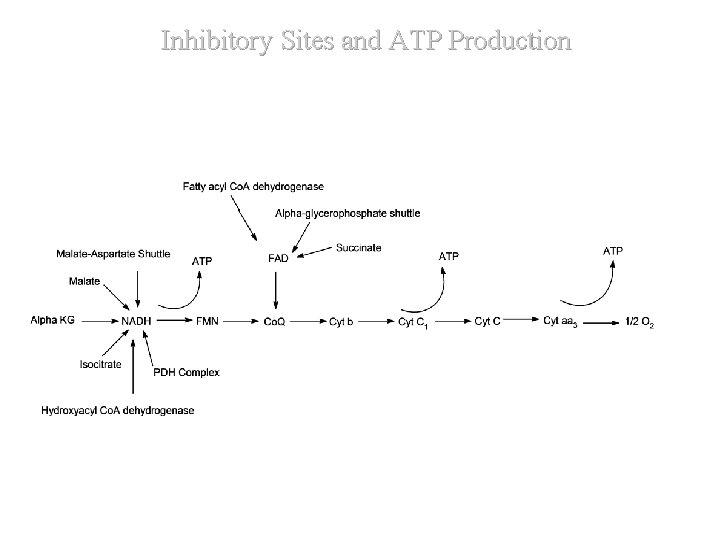
Inhibitory Sites and ATP Production
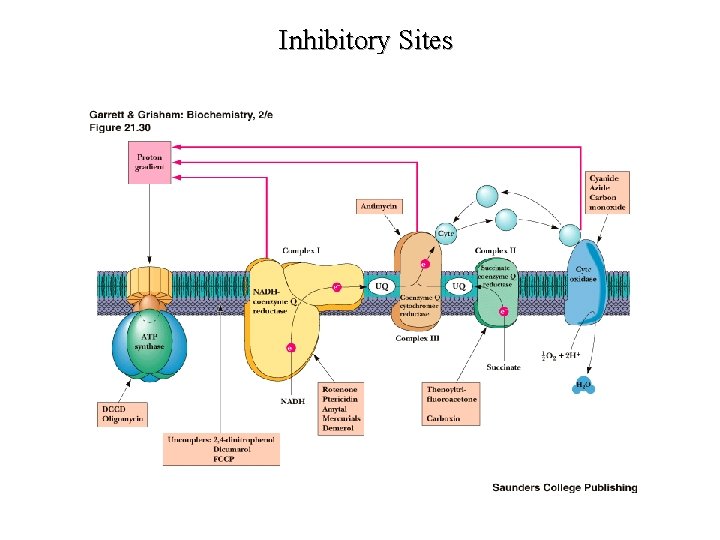
Inhibitory Sites
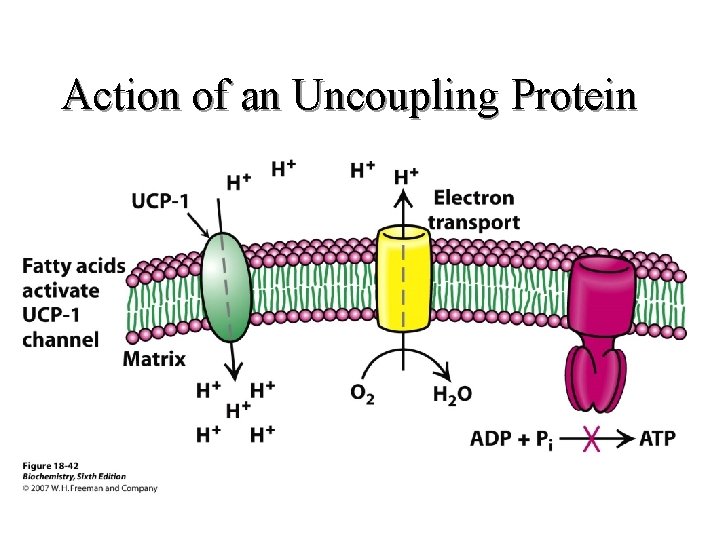
Action of an Uncoupling Protein
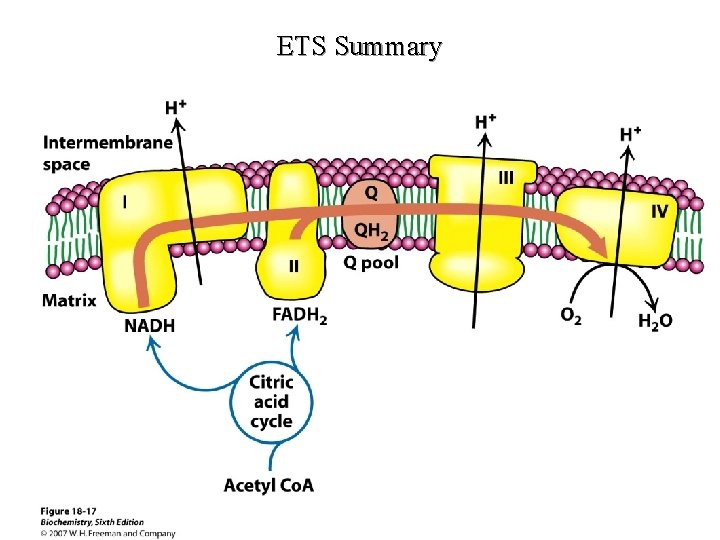
ETS Summary
- Slides: 75