Electron Structure By Diane Paskowski Most Important Ideas

Electron Structure By Diane Paskowski
Most Important Ideas of Quantum Mechanics n Orbital is not an orbit n Electron NOT moving around the nucleus n DO NOT KNOW how it is moving
What does it all mean? n n n Wave function has no easily visualized physical meaning Square of the function indicates the probability of finding an electron near a particular point in space Probabilty distribution

Relative Orbital Size n n Difficult to define precisely. Orbital is a wave function. Picture an orbital as a three-dimensional electron density map. Hydrogen 1 s orbital: n Radius of the sphere that encloses 90% of the total electron probability. 7. 5
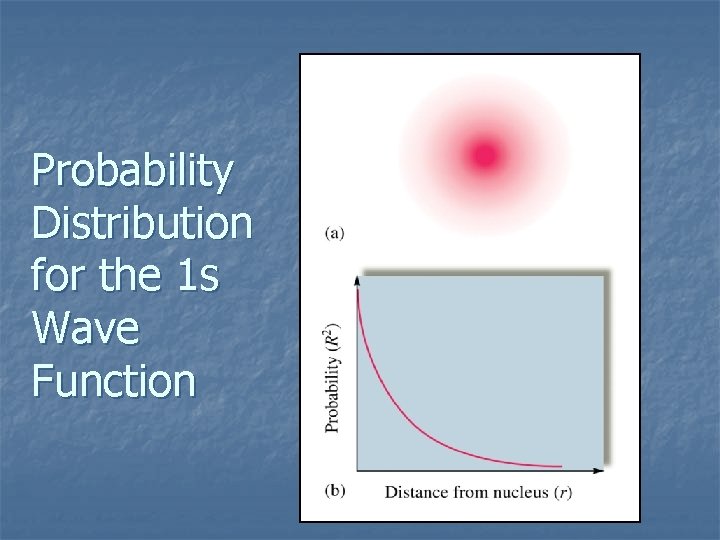
Probability Distribution for the 1 s Wave Function
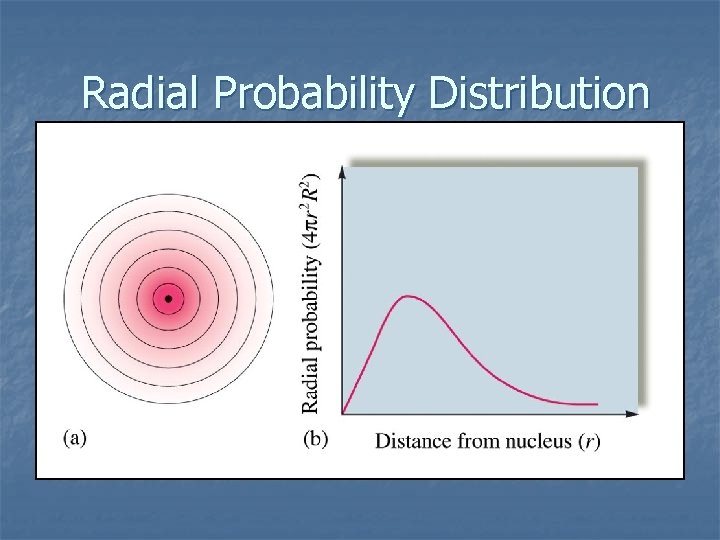
Radial Probability Distribution

Quantum Numbers n n Three “variables” contained within the wave function – n, l, ml First, the value of n determines the possible values of l The values of l, then determine the possible values of ml The magnetic spin, ms, is not determined by the wave function
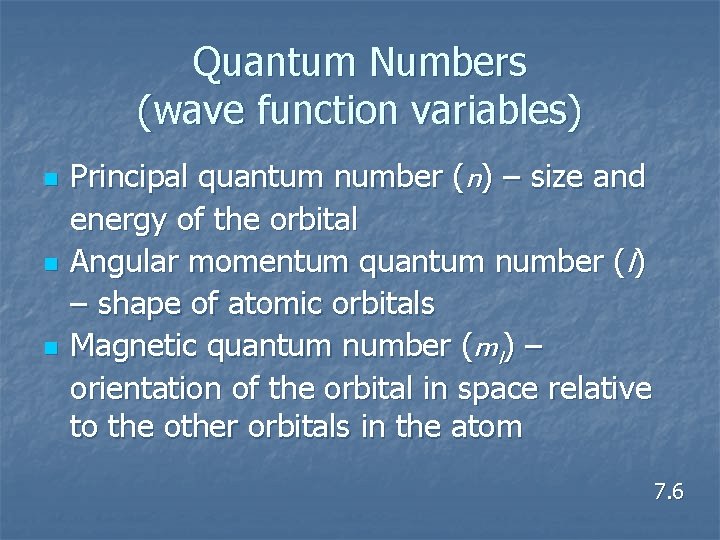
Quantum Numbers (wave function variables) n n n Principal quantum number (n) – size and energy of the orbital Angular momentum quantum number (l) – shape of atomic orbitals Magnetic quantum number (ml) – orientation of the orbital in space relative to the other orbitals in the atom 7. 6

Table 7. 1 The Angular Momentum Quantum Numbers (l) and Corresponding Letters Used to Designate Atomic Orbitals
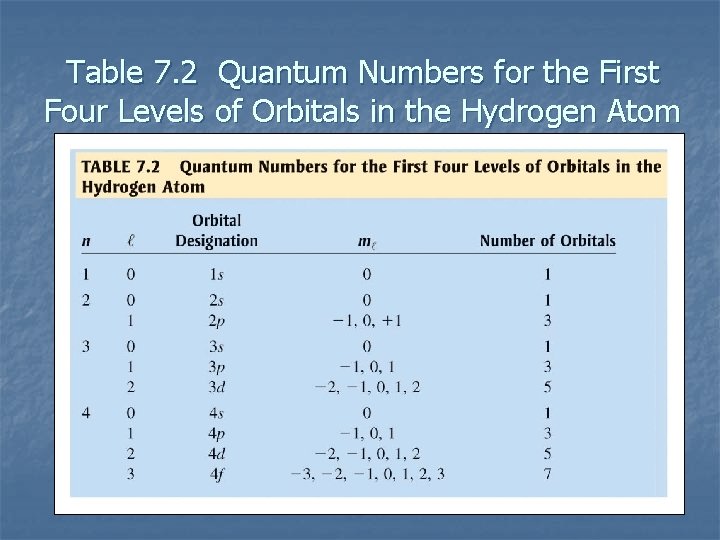
Table 7. 2 Quantum Numbers for the First Four Levels of Orbitals in the Hydrogen Atom
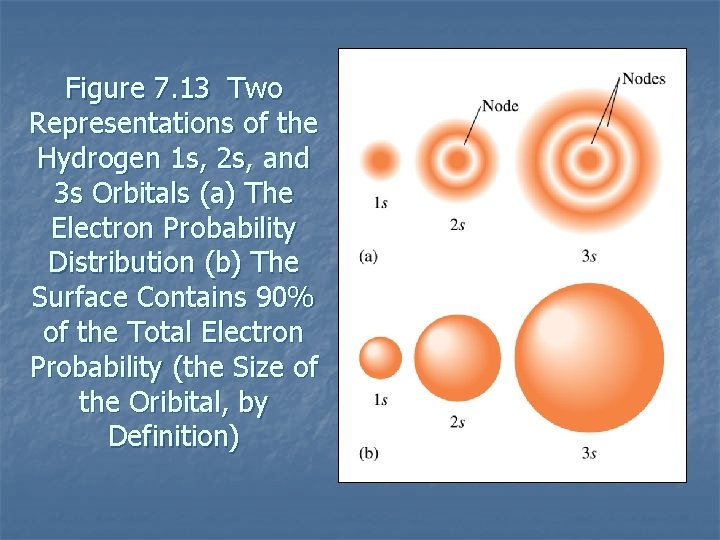
Figure 7. 13 Two Representations of the Hydrogen 1 s, 2 s, and 3 s Orbitals (a) The Electron Probability Distribution (b) The Surface Contains 90% of the Total Electron Probability (the Size of the Oribital, by Definition)
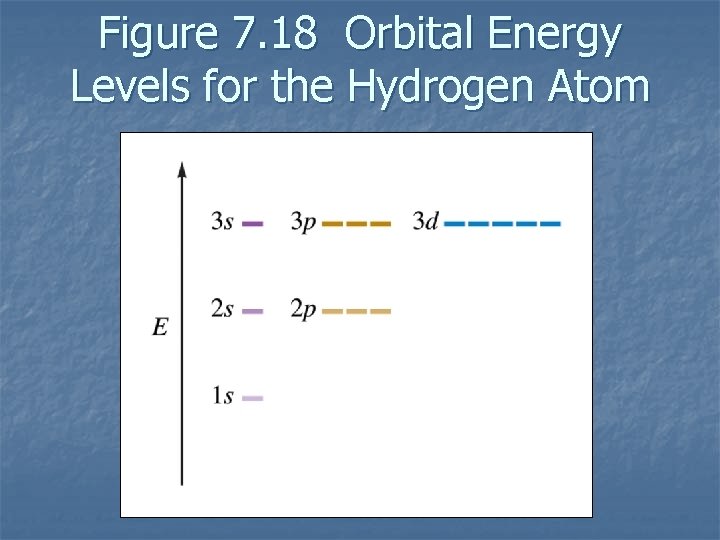
Figure 7. 18 Orbital Energy Levels for the Hydrogen Atom
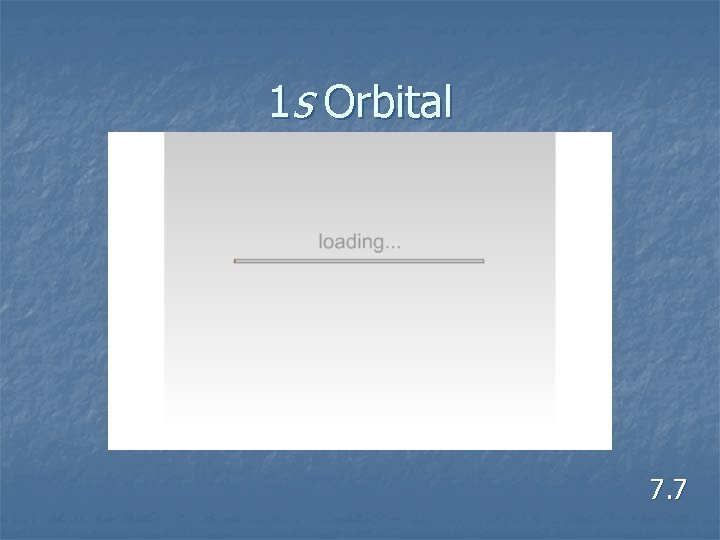
1 s Orbital 7. 7

2 px Orbital 7. 7
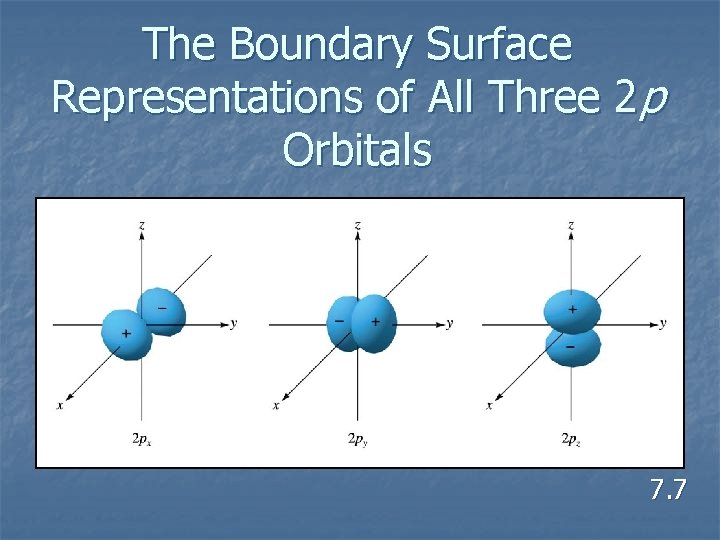
The Boundary Surface Representations of All Three 2 p Orbitals 7. 7
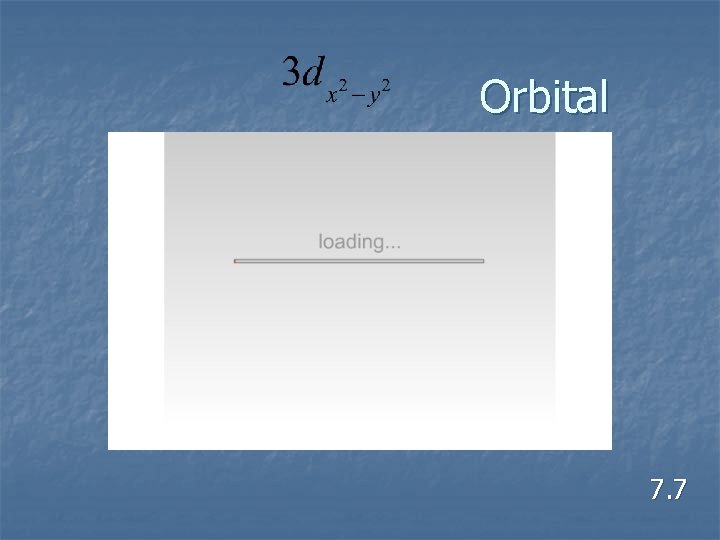
Orbital 7. 7

3 dxy Orbital 7. 7
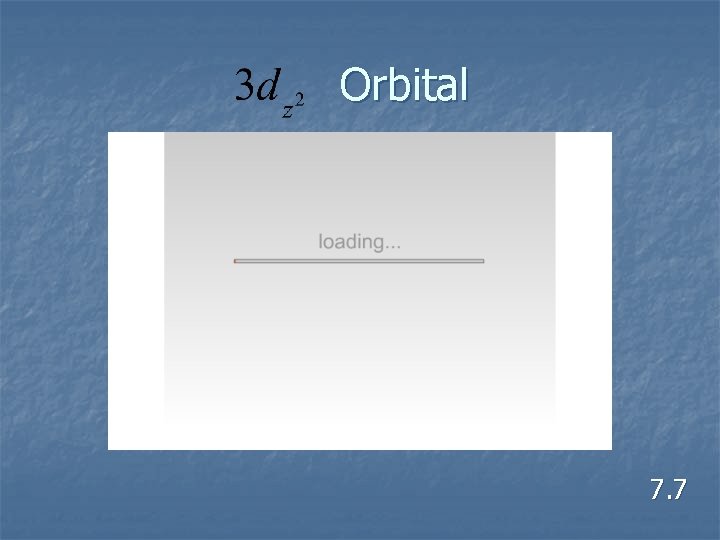
Orbital 7. 7
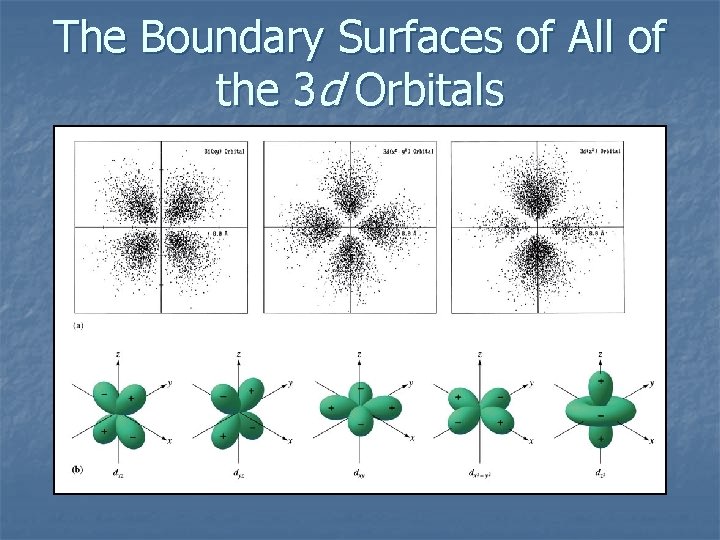
The Boundary Surfaces of All of the 3 d Orbitals
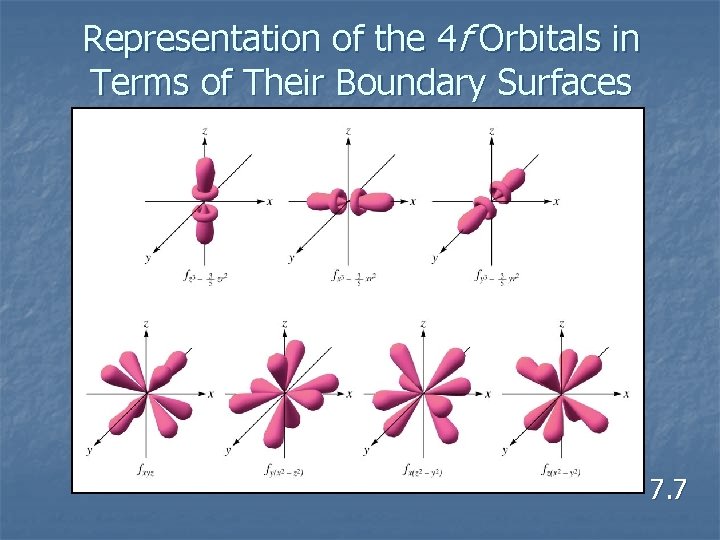
Representation of the 4 f Orbitals in Terms of Their Boundary Surfaces 7. 7

Electron Spin n Electron spin quantum number (ms) – can be +½ or -½. Pauli exclusion principle - in a given atom no two electrons can have the same set of four quantum numbers. An orbital can hold only two electrons, and they must have opposite spins. 7. 8
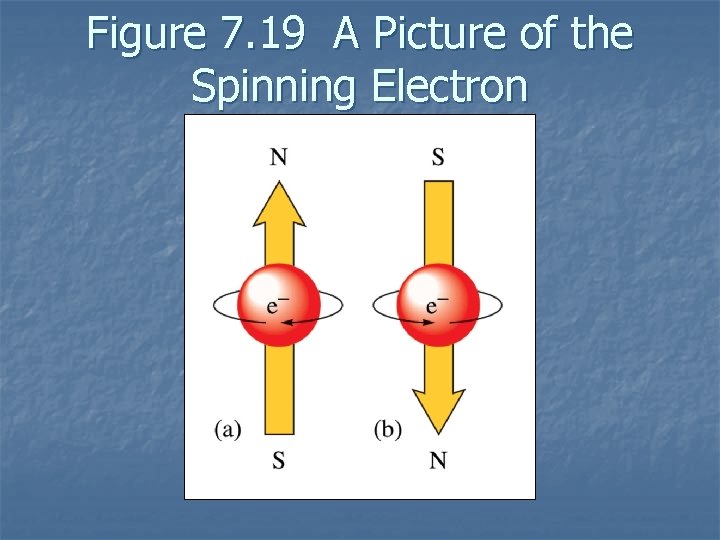
Figure 7. 19 A Picture of the Spinning Electron
Orbital Energies 7. 9
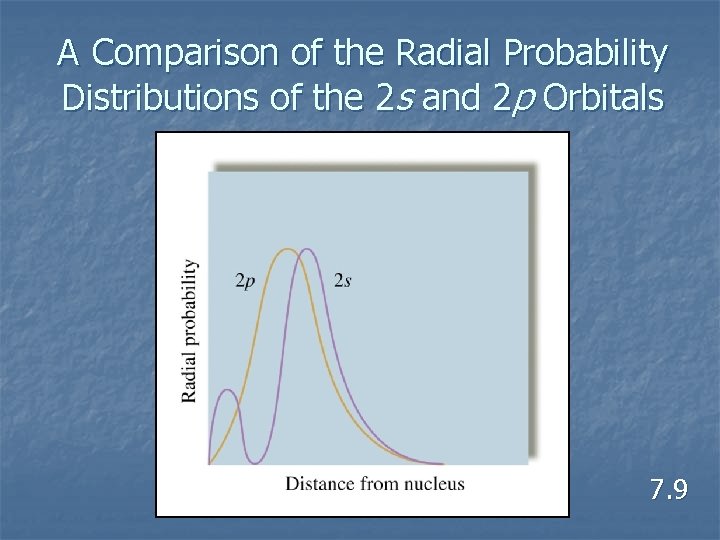
A Comparison of the Radial Probability Distributions of the 2 s and 2 p Orbitals 7. 9

The Radial Probability Distribution of the 3 s Orbital 7. 9
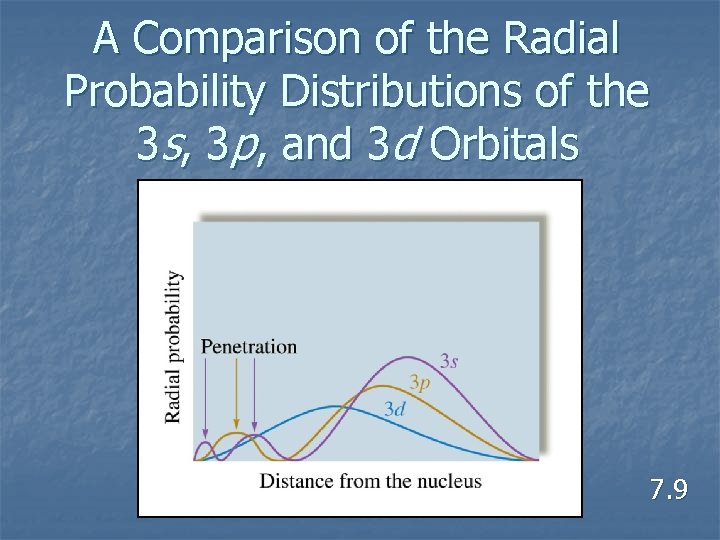
A Comparison of the Radial Probability Distributions of the 3 s, 3 p, and 3 d Orbitals 7. 9
Polyelectronic Atoms n Electron correlation problem: n n Since the electron pathways are unknown, the electron repulsions cannot be calculated exactly When electrons are placed in a particular quantum level, they “prefer” the orbitals in the order s, p, d, and then f. 7. 9
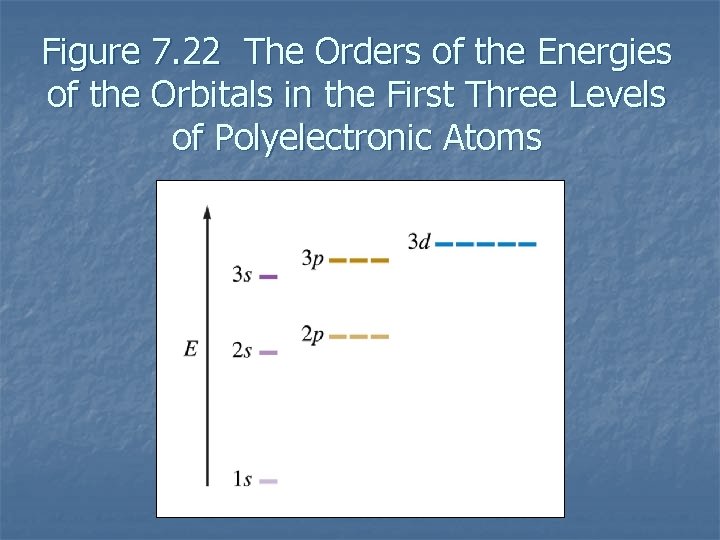
Figure 7. 22 The Orders of the Energies of the Orbitals in the First Three Levels of Polyelectronic Atoms

Aufbau Principle n As protons are added one by one to the nucleus to build up the elements, electrons are similarly added to hydrogen like orbitals. 7. 11

Hund’s Rule n The lowest energy configuration for an atom is the one having the maximum number of unpaired electrons allowed by the Pauli principle in a particular set of degenerate orbitals. 7. 11
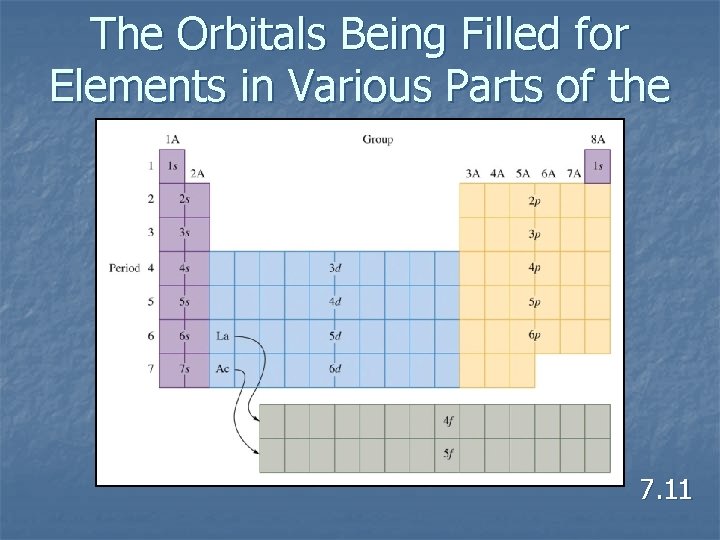
The Orbitals Being Filled for Elements in Various Parts of the Periodic Table 7. 11
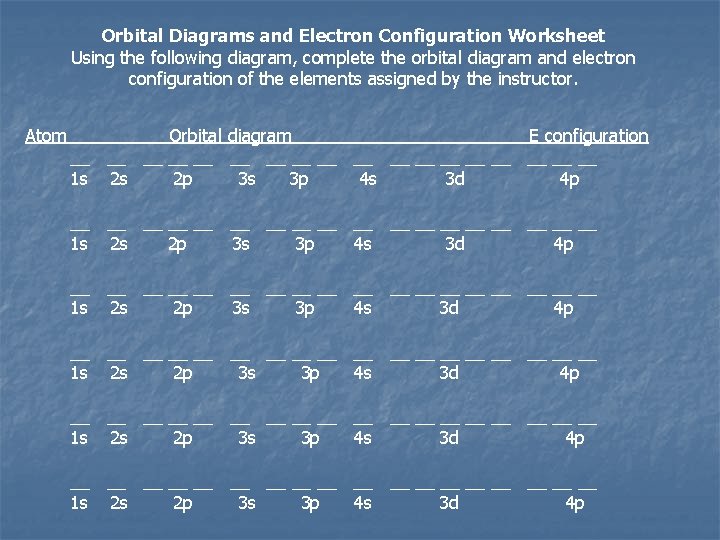
Orbital Diagrams and Electron Configuration Worksheet Using the following diagram, complete the orbital diagram and electron configuration of the elements assigned by the instructor. Atom Orbital diagram E configuration __ __ __ __ __ __ __ __ __ __ __ __ __ __ __ __ __ __ 1 s 2 s 2 p 3 s 3 p 4 s 3 d 4 p __ __ __ __ __ __ __ __ __ 1 s 2 s 2 p 3 s 3 p 4 s 3 d 4 p

Valence Electrons n n The electrons in the outermost principal quantum level of an atom. 1 s 22 p 6 (valence electrons = 8) The elements in the same group on the periodic table have the same valence electron configuration. 7. 11

Exercise n Determine the expected electron configurations for each of the following. a) S b) Ba c) Eu 7. 11

History of the Periodic Table n n Many early scientists organized the elements in a table. Mendeleev organized the elements according to physical and chemical properties. n Correctly predicted the existence of previously undiscovered elements and their properties n
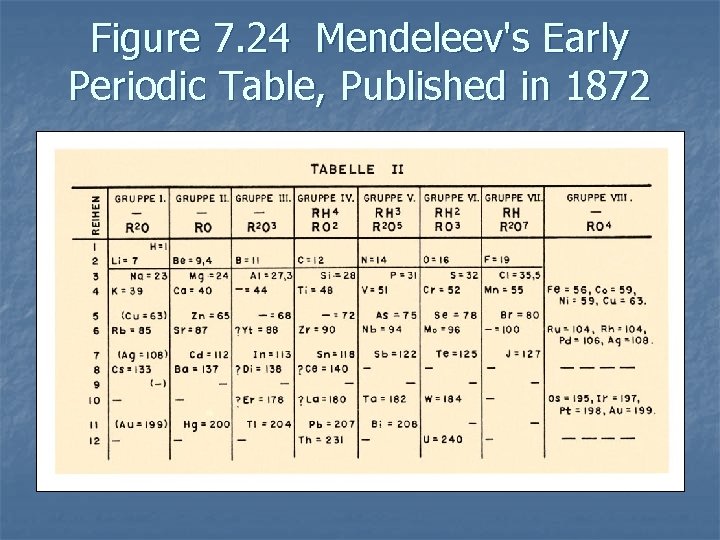
Figure 7. 24 Mendeleev's Early Periodic Table, Published in 1872

Periodic Trends n n n Ionization Energy Electron Affinity Atomic Radius 7. 12

Ionization Energy n n n Energy required to remove an electron from a gaseous atom or ion. In general, as we go across a period from left to right, the first ionization energy increases. In general, as we go down a group from top to bottom, the first ionization energy decreases. 7. 12

Ionization Energy n Why does the IE decrease down a group? n Why does the IE increase across a period? n n The effective nuclear charge increases across a period and decreases down a column. What is effective nuclear charge?
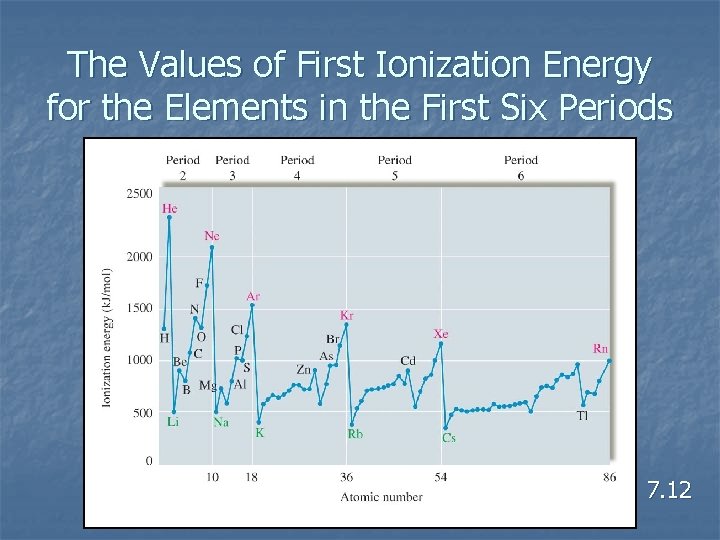
The Values of First Ionization Energy for the Elements in the First Six Periods 7. 12

Concept Check n n Explain why the graph of ionization energy versus atomic number (across a row) is not linear. Where are the exceptions? 7. 12
Concept Check n Which atom would require more energy to remove an electron? Why? Li Cs 7. 12

Concept Check n Which has the larger second ionization energy? Why? Lithium or Beryllium 7. 12
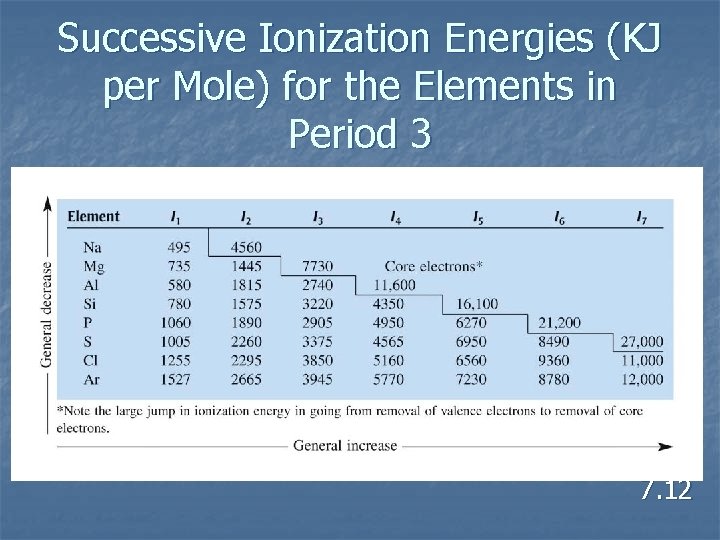
Successive Ionization Energies (KJ per Mole) for the Elements in Period 3 7. 12

Electron Affinity n n n Energy change associated with the addition of an electron to a gaseous atom. In general as we go across a period from left to right, the electron affinities become more negative. In general electron affinity becomes more positive in going down a group. 7. 12
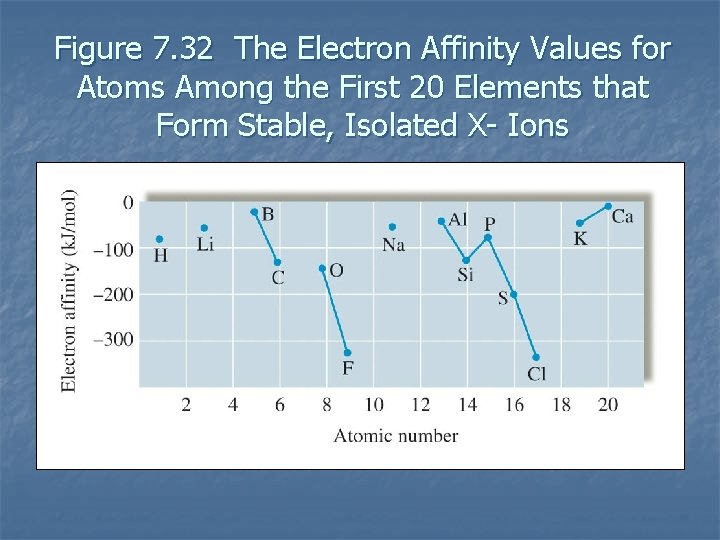
Figure 7. 32 The Electron Affinity Values for Atoms Among the First 20 Elements that Form Stable, Isolated X- Ions

Atomic Radius n n In general as we go across a period from left to right, the atomic radius decreases. In general atomic radius increases in going down a group. 7. 12
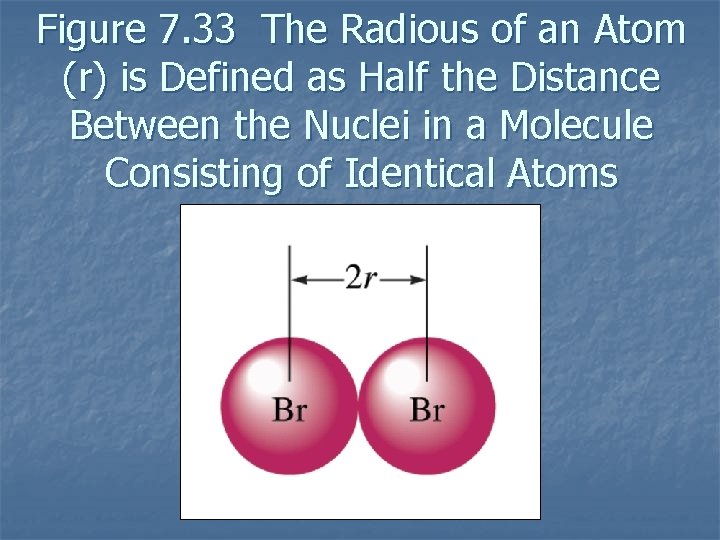
Figure 7. 33 The Radious of an Atom (r) is Defined as Half the Distance Between the Nuclei in a Molecule Consisting of Identical Atoms

Concept Check n Which should be the larger atom? Why? Na Cl 7. 12

Concept Check Which is larger? n The hydrogen 1 s orbital n The lithium 1 s orbital Which is lower in energy? n The hydrogen 1 s orbital n The lithium 1 s orbital 7. 12

Atomic Radius of a Metal 7. 12

Atomic Radius of a Nonmetal 7. 12
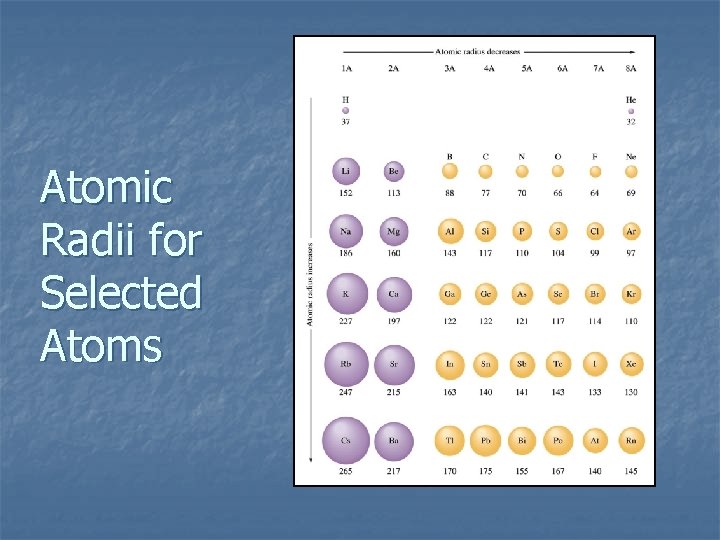
Atomic Radii for Selected Atoms

Exercise n Arrange the elements oxygen, fluorine, and sulfur according to increasing: n Ionization energy n Atomic size 7. 12

Final Thoughts n n It is the number of valence electrons that chemists use to explain an atom’s chemistry. BUT do not forget the involvement of the nucleus. n Electrostatic interaction of positive and negative charges is the fundamental force that explains chemical interactions. n i. e. , the nucleus of one atom MUST attract the electrons of another atom to create a chemical bond. . . 7. 13
- Slides: 55