ELECTRON ORBITALS Cartoon courtesy of labinitio com THE
ELECTRON ORBITALS Cartoon courtesy of lab-initio. com
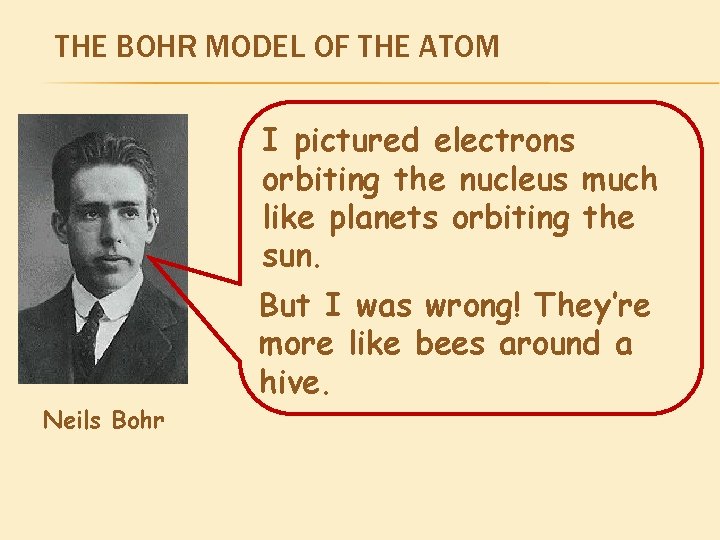
THE BOHR MODEL OF THE ATOM I pictured electrons orbiting the nucleus much like planets orbiting the sun. Neils Bohr But I was wrong! They’re more like bees around a hive.
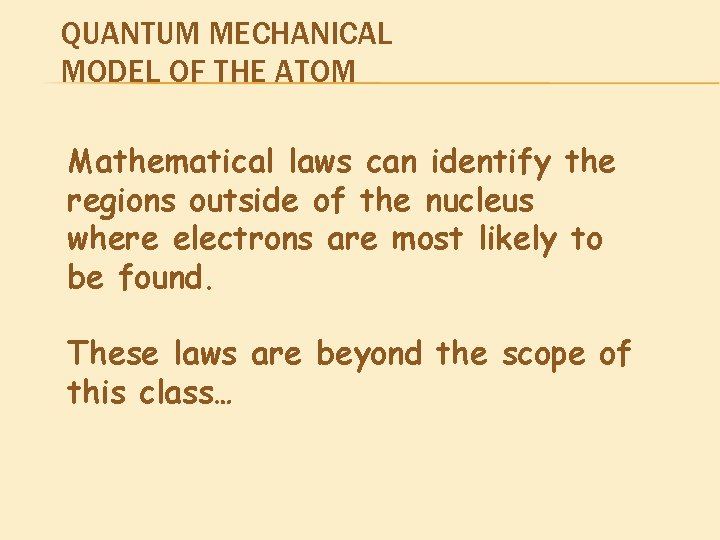
QUANTUM MECHANICAL MODEL OF THE ATOM Mathematical laws can identify the regions outside of the nucleus where electrons are most likely to be found. These laws are beyond the scope of this class…

HEISENBERG UNCERTAINTY PRINCIPLE “One cannot simultaneously determine both the position and momentum of an electron. ” You can find out where the electron is, but not where it is going. Werner Heisenberg OR… You can find out where the electron is going, but not where it is!
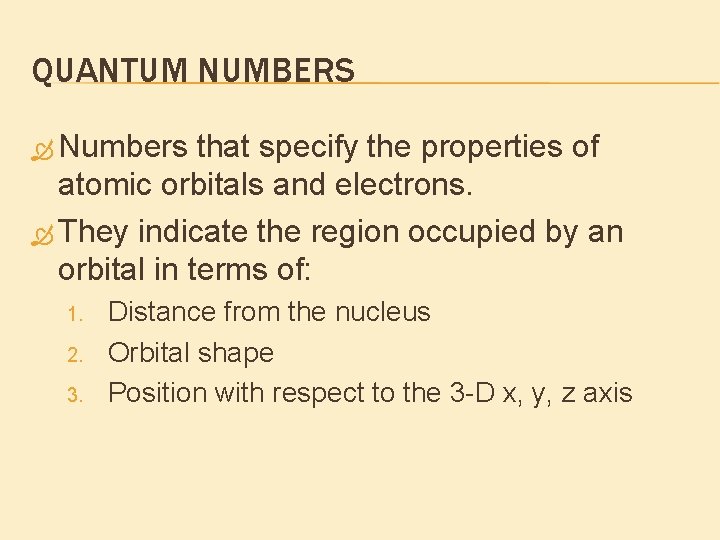
QUANTUM NUMBERS Numbers that specify the properties of atomic orbitals and electrons. They indicate the region occupied by an orbital in terms of: 1. 2. 3. Distance from the nucleus Orbital shape Position with respect to the 3 -D x, y, z axis
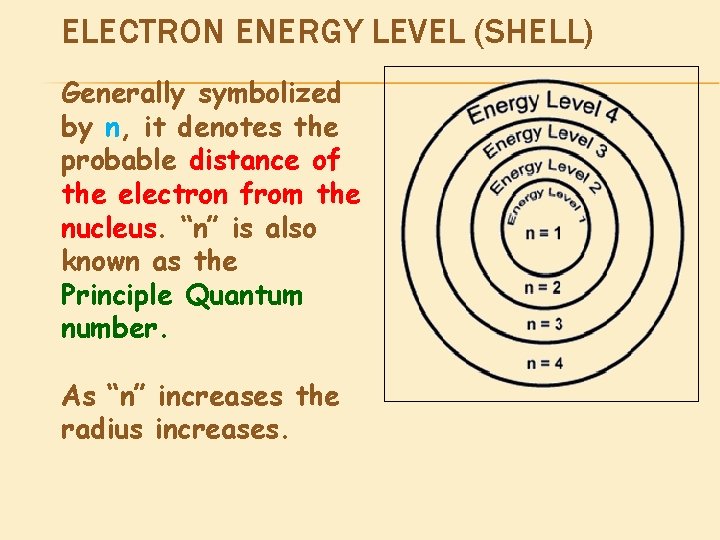
ELECTRON ENERGY LEVEL (SHELL) Generally symbolized by n, it denotes the probable distance of the electron from the nucleus. “n” is also known as the Principle Quantum number. As “n” increases the radius increases.
ORBITAL QUNATUM NUMBER (SUBSHELL) Generally symbolized by l, it is a measure of orbital angular momentum, which indicates the shape of the orbital.
MAGNETIC QUANTUM NUMBER Generally symbolized by ml, in indicates the orbital around the 3 axes in space (orientation in space) s = 1 orientation p = 3 orientations d = 5 orientations f = 7 orientations Identifies the specific orbital.

SPIN QUANTUM NUMBER Generally symbolized by ms, it tells the electrons spin on its axis. Clockwise or counterclockwise Whether bound or free all electrons spin.

Electron Orbitals AN ORBITAL IS A REGION WITHIN AN ENERGY LEVEL WHERE THERE IS A PROBABILITY OF FINDING AN ELECTRON. Orbital shapes are defined as the surface that contains 90% of the total electron probability.

There are four shapes or sublevels: s p d f Each sublevel has “orientations” or orbitals around the origin of the x-y-z axis. Take a look!
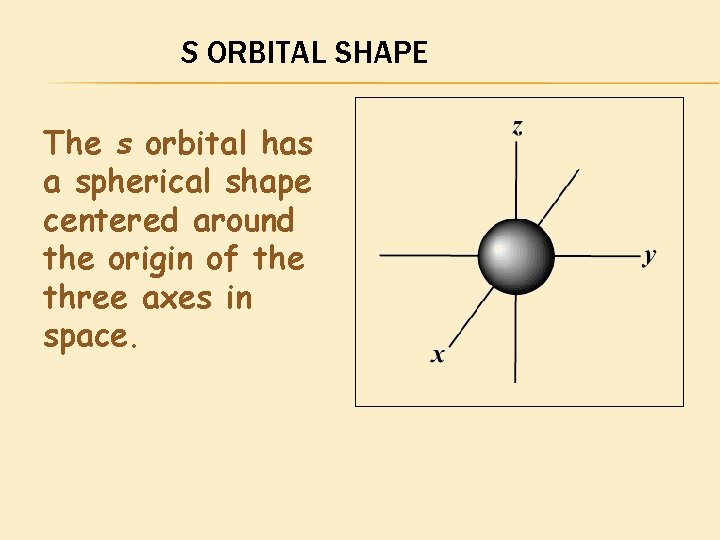
S ORBITAL SHAPE The s orbital has a spherical shape centered around the origin of the three axes in space.
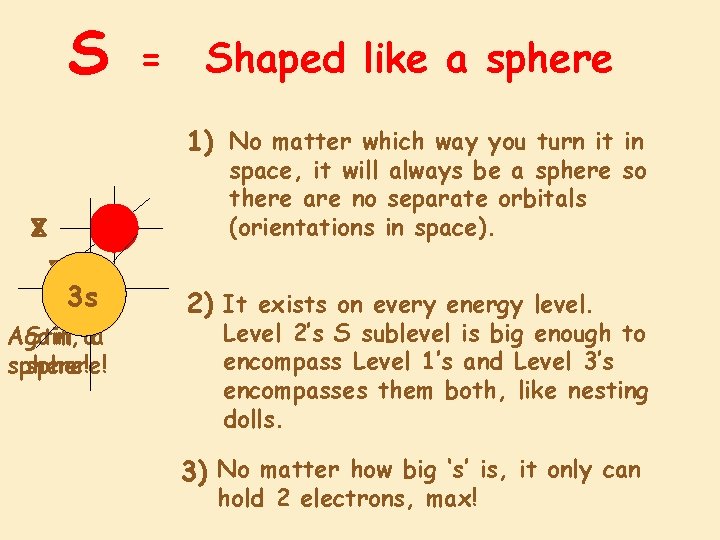
S = Shaped like a sphere 1) No matter which way you turn it in space, it will always be a sphere so there are no separate orbitals (orientations in space). X Z Y X 1 s Z 2 s 3 s Y X Again, Still aa sphere! 2) It exists on every energy level. Level 2’s S sublevel is big enough to encompass Level 1’s and Level 3’s encompasses them both, like nesting dolls. 3) No matter how big ‘s’ is, it only can hold 2 electrons, max!
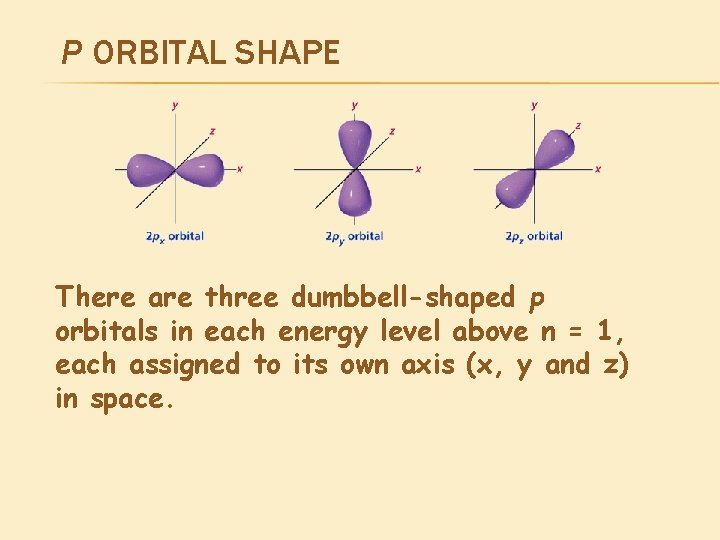
P ORBITAL SHAPE There are three dumbbell-shaped p orbitals in each energy level above n = 1, each assigned to its own axis (x, y and z) in space.
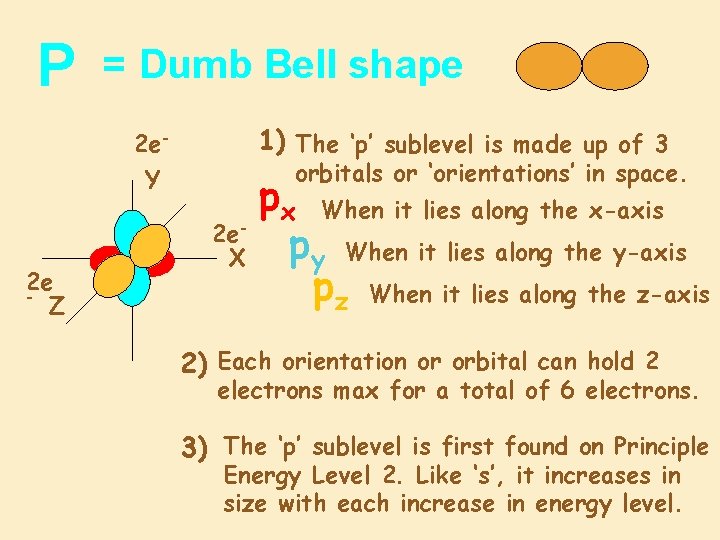
P = Dumb Bell shape 1) The ‘p’ sublevel is made up of 3 2 e. Y 2 e Z orbitals or ‘orientations’ in space. 2 e. X px When it lies along the x-axis py When it lies along the y-axis pz When it lies along the z-axis 2) Each orientation or orbital can hold 2 electrons max for a total of 6 electrons. 3) The ‘p’ sublevel is first found on Principle Energy Level 2. Like ‘s’, it increases in size with each increase in energy level.
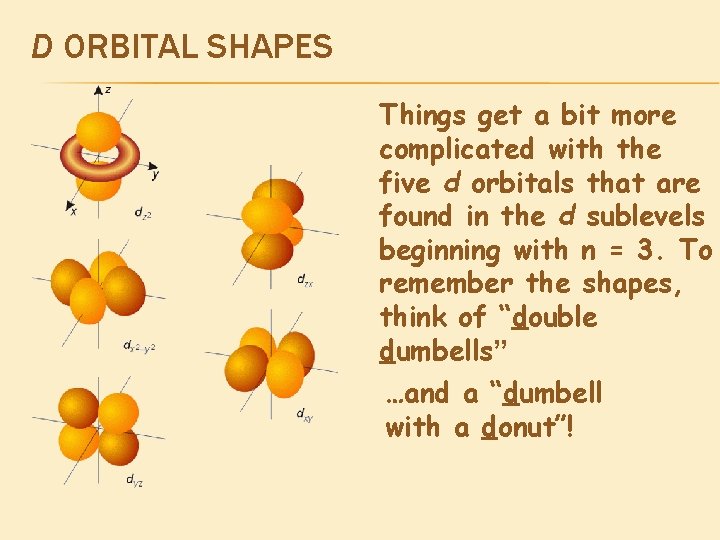
D ORBITAL SHAPES Things get a bit more complicated with the five d orbitals that are found in the d sublevels beginning with n = 3. To remember the shapes, think of “double dumbells” …and a “dumbell with a donut”!
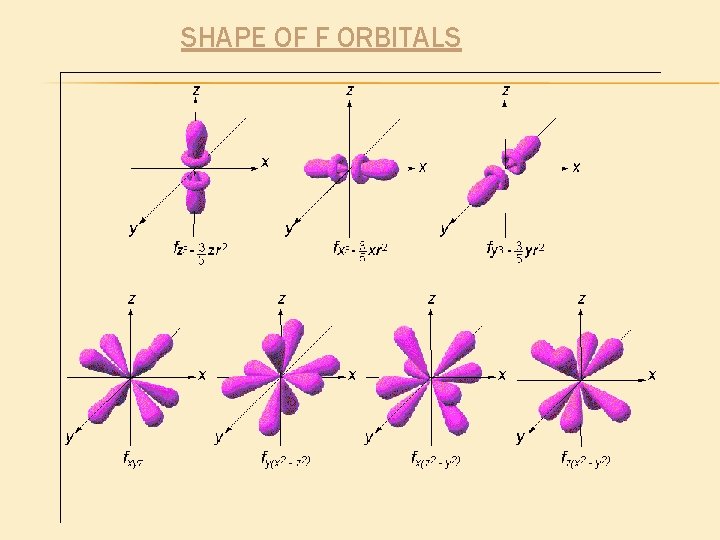
SHAPE OF F ORBITALS
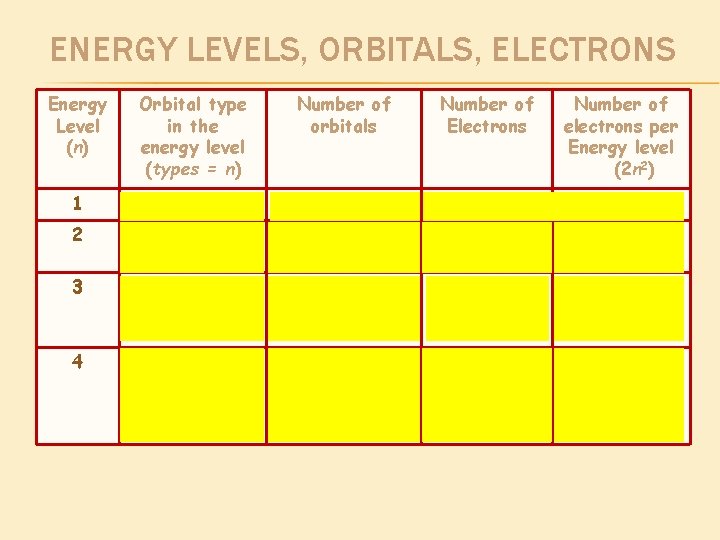
ENERGY LEVELS, ORBITALS, ELECTRONS Energy Level (n) Orbital type in the energy level (types = n) Number of orbitals Number of Electrons Number of electrons per Energy level (2 n 2) 1 s 1 2 2 2 s p 1 3 2 6 8 3 s p d 1 3 5 2 6 10 18 4 s p d f 1 3 5 7 2 6 10 14 32
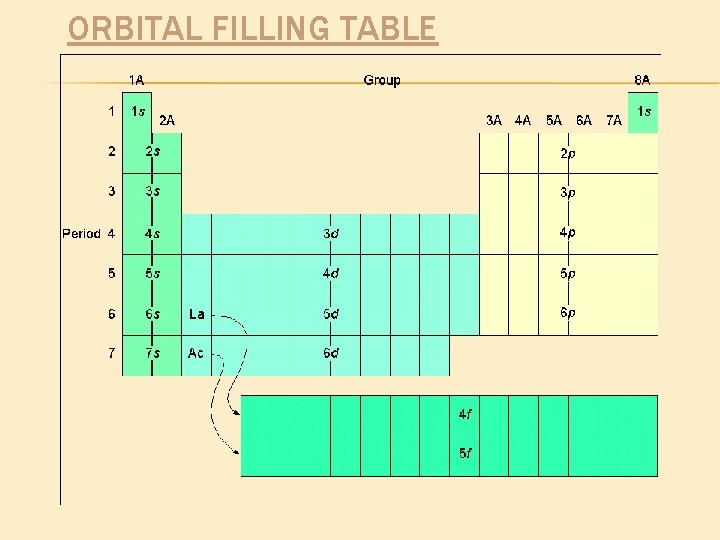
ORBITAL FILLING TABLE
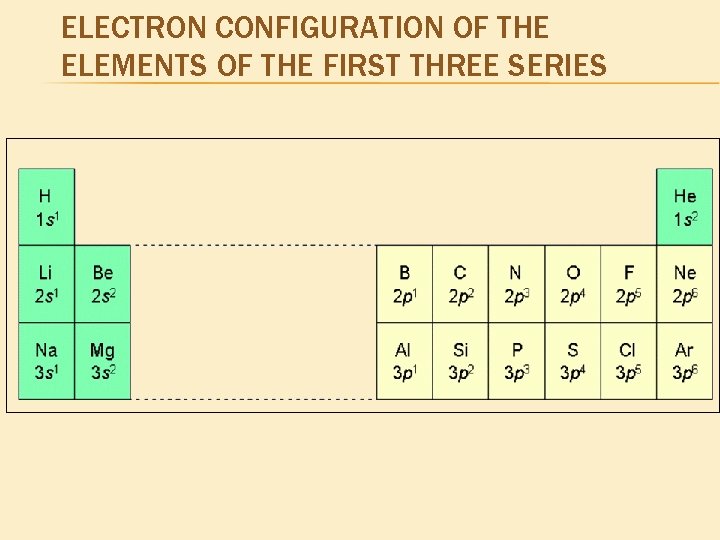
ELECTRON CONFIGURATION OF THE ELEMENTS OF THE FIRST THREE SERIES
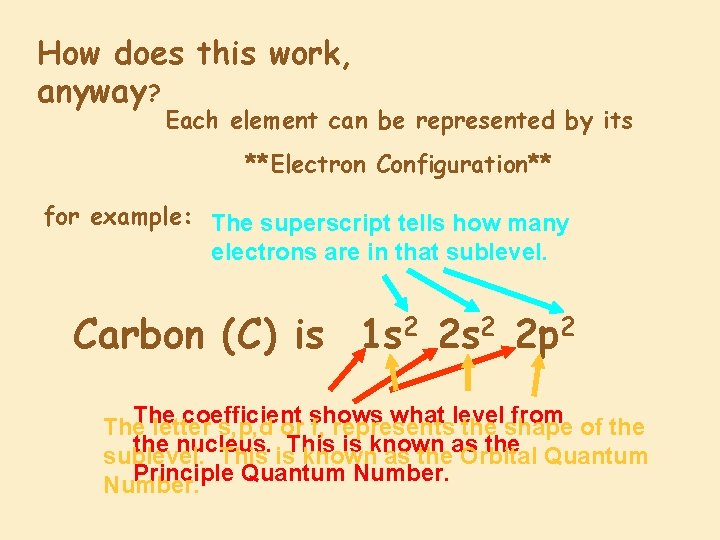
How does this work, anyway? Each element can be represented by its **Electron Configuration** for example: The superscript tells how many electrons are in that sublevel. Carbon (C) is 1 s 2 2 p 2 The coefficient what level from of the The letter s, p, d or shows f, represents the shape the nucleus. is known the Quantum sublevel. This is. This known as theas Orbital Principle Quantum Number.

So There are 4 electrons total in the valence shell. These are the valence electrons. 1 s 22 p 2 tells us that Carbon has: 2 2 2 electrons in the ‘s’ sublevel (the sphere) on principle energy level ‘ 1’ The outermost energy level is ‘ 2’ for Carbon. This is its valence shell. 2 electrons in the ‘s’ sublevel (the sphere) on principle energy level ‘ 2’ 2 electrons in the ‘p’ sublevel (the dumb bell) on principle energy level ‘ 2’
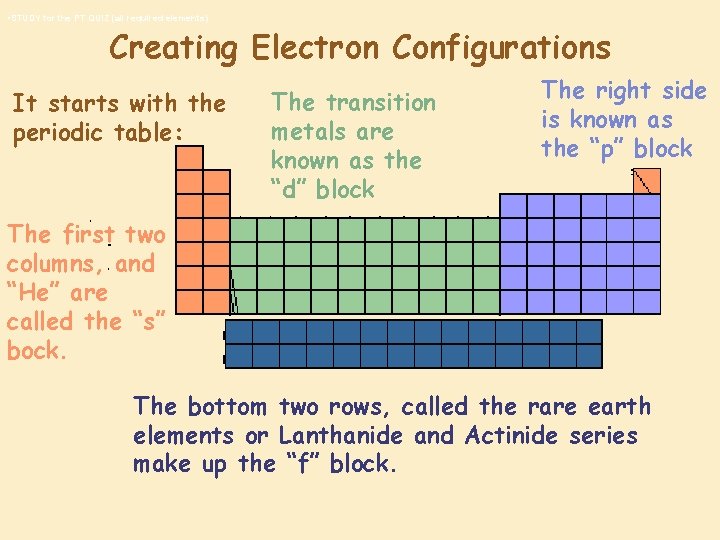
• STUDY for the PT QUIZ (all required elements) Creating Electron Configurations It starts with the periodic table: The transition metals are known as the “d” block The right side is known as the “p” block The first two columns, and “He” are called the “s” bock. The bottom two rows, called the rare earth elements or Lanthanide and Actinide series make up the “f” block.
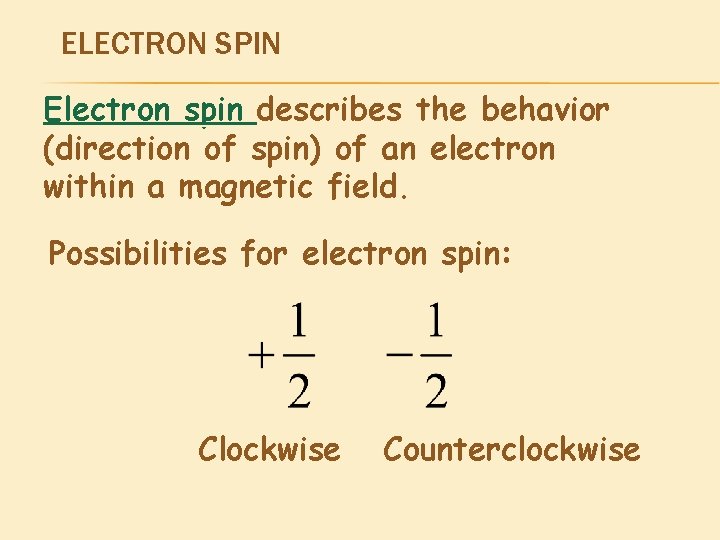
ELECTRON SPIN Electron spin describes the behavior (direction of spin) of an electron within a magnetic field. Possibilities for electron spin: Clockwise Counterclockwise

PAULI EXCLUSION PRINCIPLE Two electrons occupying the same orbital must have opposite spins Wolfgang Pauli
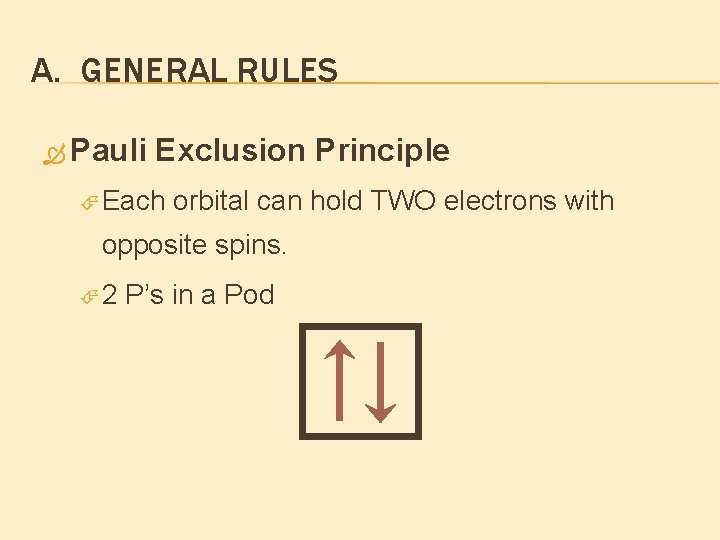
A. GENERAL RULES Pauli Exclusion Principle Each orbital can hold TWO electrons with opposite spins. 2 P’s in a Pod
A. GENERAL RULES Aufbau Principle Electrons fill the lowest energy orbitals first. “Lazy Rule” au Tenant f. B A U

A. GENERAL RULES Hund’s Rule Within a sublevel, place one e- per orbital before pairing them. “Empty Bus Seat Rule” or Hand’s Rule WRONG RIGHT
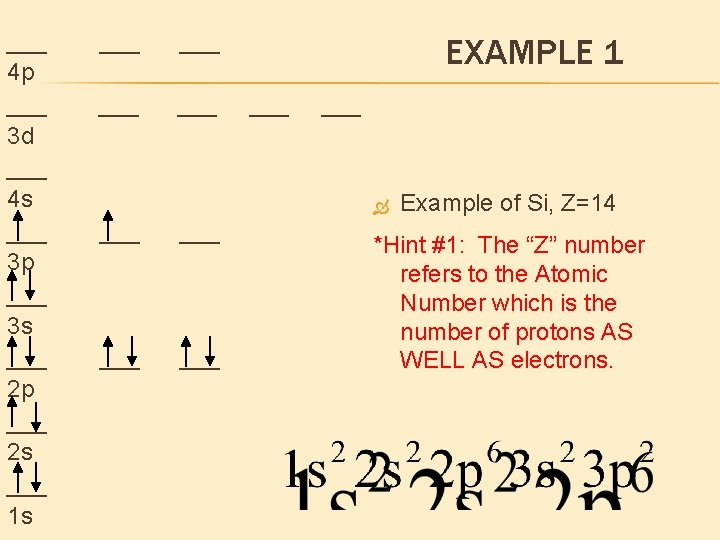
___ 4 p ___ 3 d ___ 4 s ___ 3 p ___ 3 s ___ 2 p ___ 2 s ___ 1 s ___ ___ EXAMPLE 1 ___ ___ ___ Example of Si, Z=14 *Hint #1: The “Z” number refers to the Atomic Number which is the number of protons AS WELL AS electrons.
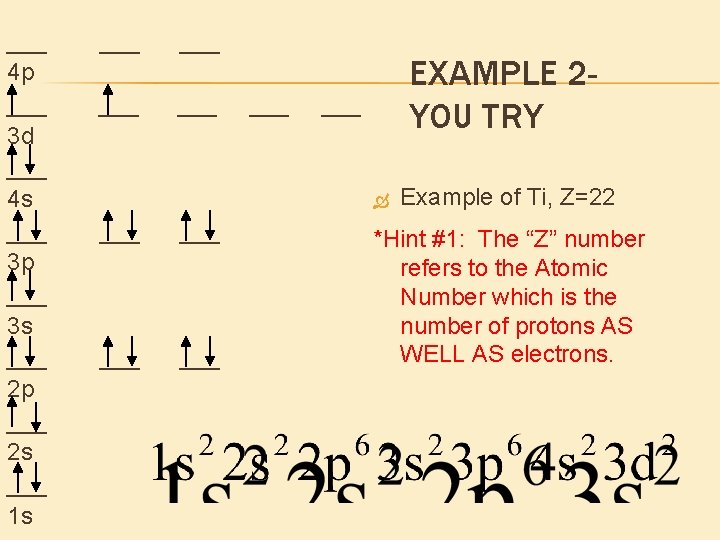
___ 4 p ___ 3 d ___ 4 s ___ 3 p ___ 3 s ___ 2 p ___ 2 s ___ 1 s ___ ___ ___ EXAMPLE 2 YOU TRY ___ ___ ___ Example of Ti, Z=22 *Hint #1: The “Z” number refers to the Atomic Number which is the number of protons AS WELL AS electrons.

YOU TRY THESE Mg Cl Co Br
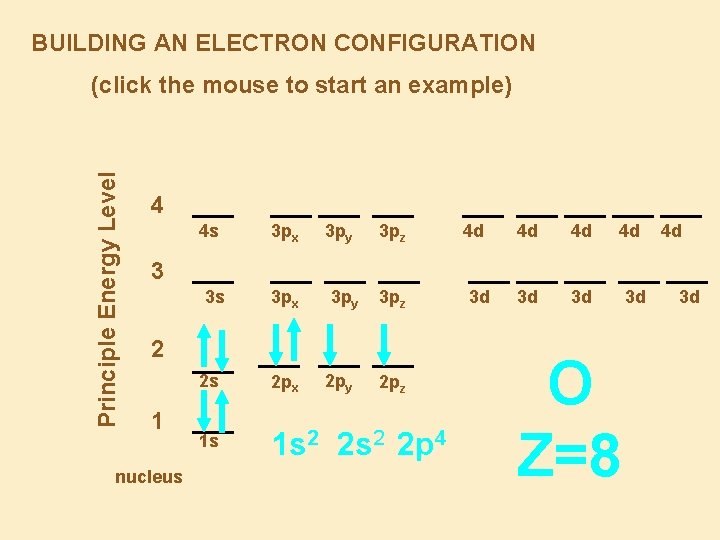
BUILDING AN ELECTRON CONFIGURATION Principle Energy Level (click the mouse to start an example) 4 4 s 3 px 3 py 3 pz 4 d 4 d 4 d 3 d 3 d 4 d 4 d 3 3 s 3 px 3 py 3 pz 2 1 nucleus 2 s 2 px 2 py 1 s 1 s 2 2 p 4 2 pz 3 d O Z=8 3 d 3 d
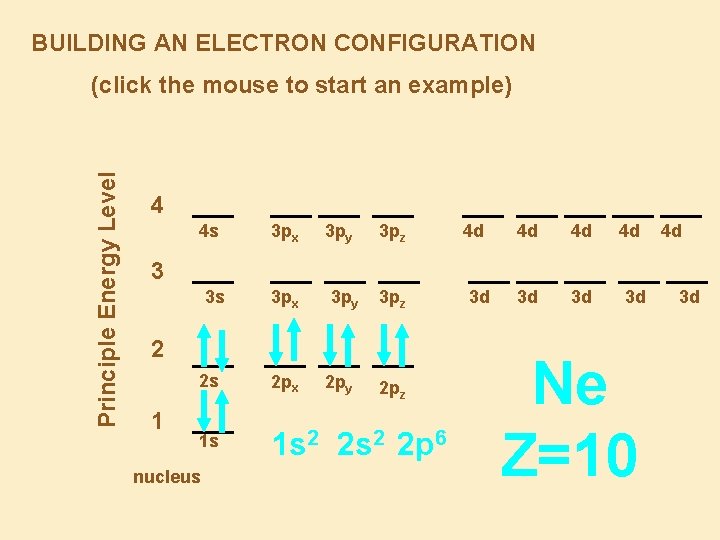
BUILDING AN ELECTRON CONFIGURATION Principle Energy Level (click the mouse to start an example) 4 4 s 3 px 3 py 3 pz 4 d 4 d 4 d 3 d 3 d 4 d 4 d 3 3 s 3 px 3 py 3 pz 2 1 2 s 2 px 1 s 1 s 2 2 p 6 nucleus 2 py 2 pz 3 d 3 d Ne Z=10 3 d
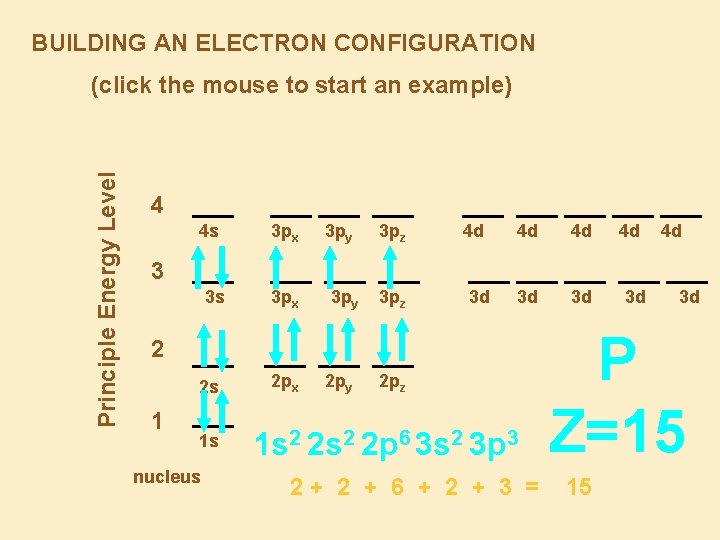
BUILDING AN ELECTRON CONFIGURATION Principle Energy Level (click the mouse to start an example) 4 4 s 3 px 3 py 3 pz 4 d 4 d 4 d 3 d 3 d 4 d 4 d 3 3 s 3 px 3 py 3 pz 3 d 2 2 s 1 1 s nucleus 2 px 2 py 2 pz 1 s 2 2 p 6 3 s 2 3 p 3 2+ 2 + 6 + 2 + 3 = 3 d 3 d P Z=15 15
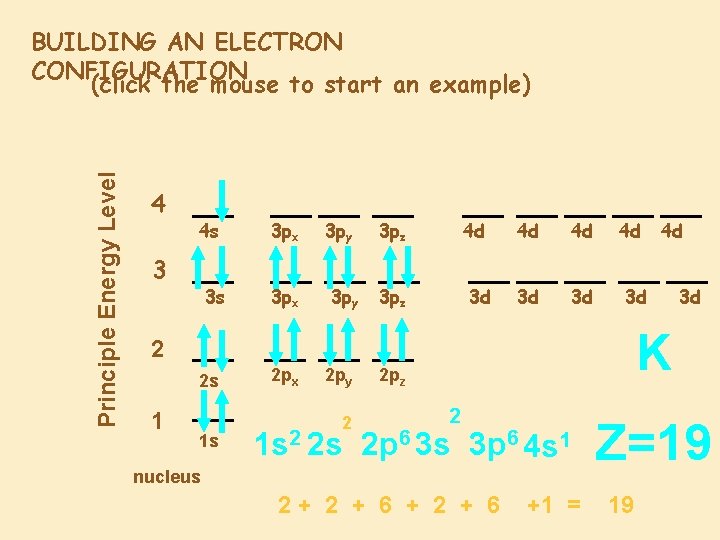
Principle Energy Level BUILDING AN ELECTRON CONFIGURATION (click the mouse to start an example) 4 4 s 3 3 s 3 px 3 py 3 pz 4 d 3 pz 3 d 4 d 4 d 3 d 3 d 4 d 3 d 2 s 1 1 s 2 py 2 3 d K 2 2 px 4 d 2 pz 2 1 s 2 2 s 2 p 6 3 s 3 p 6 4 s 1 nucleus 2+ 2 + 6 +1 = Z=19 19
![Element Lithium Configuration notation Orbital notation 1 s 22 s 1 [He]2 s 1 Element Lithium Configuration notation Orbital notation 1 s 22 s 1 [He]2 s 1](http://slidetodoc.com/presentation_image_h2/453c913745f960b3117725234692946b/image-36.jpg)
Element Lithium Configuration notation Orbital notation 1 s 22 s 1 [He]2 s 1 ____ 1 s Beryllium ____ 2 p ____ 2 s ____ 2 p ____ [He]2 s 2 p 2 ____ 2 s ____ 2 p ____ 1 s 22 p 3 [He]2 s 2 p 3 ____ 2 s ____ 2 p ____ 1 s 22 p 4 [He]2 s 2 p 4 ____ 2 s ____ 2 p ____ 1 s 22 p 5 [He]2 s 2 p 5 ____ 1 s Neon ____ 2 s 1 s 22 p 2 ____ 1 s Fluorine ____ [He]2 s 2 p 1 ____ 1 s Oxygen ____ 2 p 1 s 22 p 1 ____ 1 s Nitrogen ____ [He]2 s 2 ____ 1 s Carbon ____ 2 s 1 s 22 s 2 ____ 1 s Boron Noble gas notation ____ 2 s ____ 2 p ____ 1 s 22 p 6 [He]2 s 2 p 6 ____ 1 s ____ 2 s ____ 2 p ____
ANALOGY Electron Cloud = dorm • Energy level (shell) = floor • Subshell = room • Orbital = love seat • Spin = each person
- Slides: 37