Electron Dot Formulas Chemistry 7C Electron Dot Formulas


- Slides: 15

Electron Dot Formulas Chemistry 7(C)

Electron Dot Formulas Lesson Objectives • Draw electron dot formulas – Ionic compounds – Covalent compounds

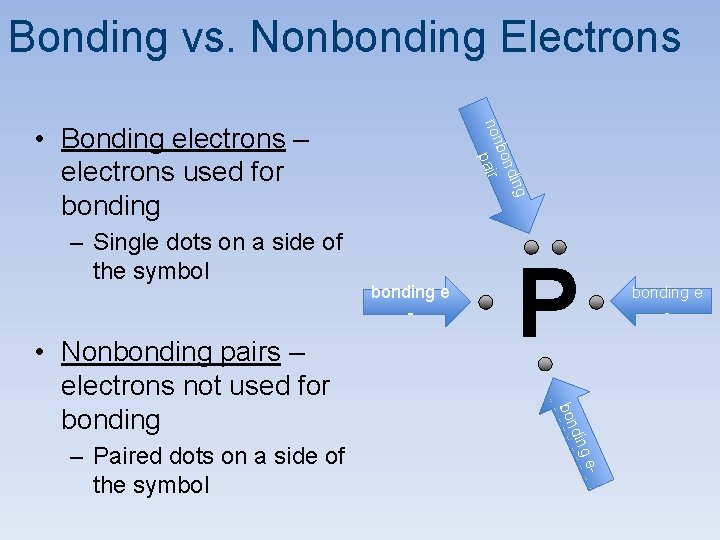
Bonding vs. Nonbonding Electrons din bon non pair – Single dots on a side of the symbol P bonding e - - ge din – Paired dots on a side of the symbol bonding e - bon • Nonbonding pairs – electrons not used for bonding g • Bonding electrons – electrons used for bonding

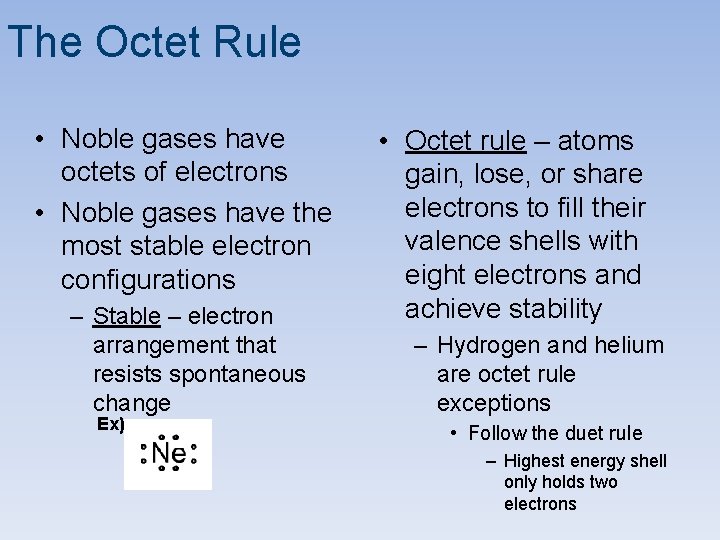
The Octet Rule • Noble gases have octets of electrons • Noble gases have the most stable electron configurations – Stable – electron arrangement that resists spontaneous change Ex) • Octet rule – atoms gain, lose, or share electrons to fill their valence shells with eight electrons and achieve stability – Hydrogen and helium are octet rule exceptions • Follow the duet rule – Highest energy shell only holds two electrons

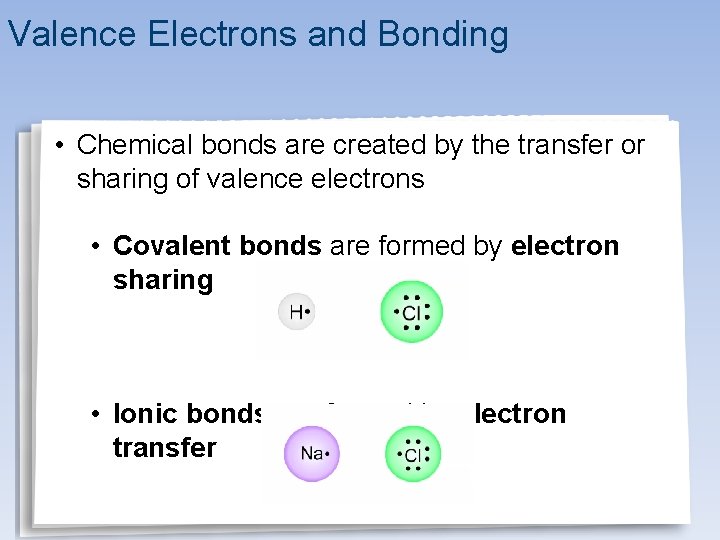
Valence Electrons and Bonding • Chemical bonds are created by the transfer or sharing of valence electrons • Covalent bonds are formed by electron sharing • Ionic bonds are formed by electron transfer

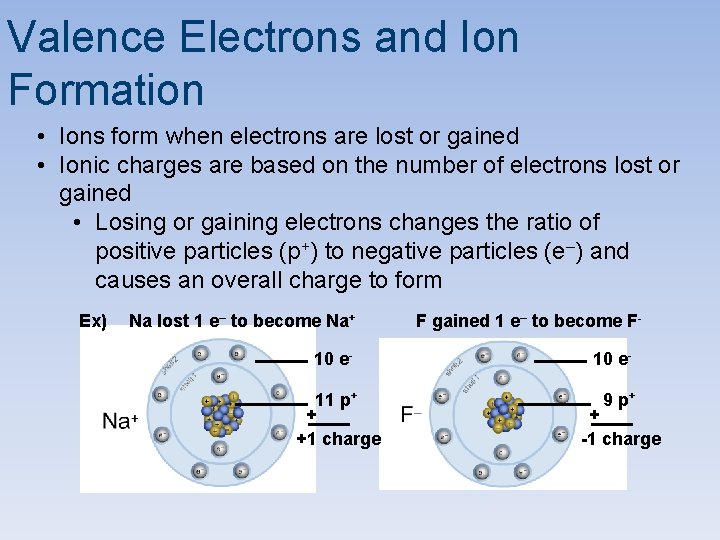
Valence Electrons and Ion Formation • Ions form when electrons are lost or gained • Ionic charges are based on the number of electrons lost or gained • Losing or gaining electrons changes the ratio of positive particles (p+) to negative particles (e–) and causes an overall charge to form Ex) Na lost 1 e– to become Na+ 10 e 11 p+ + +1 charge F gained 1 e– to become F 10 e 9 p+ + -1 charge

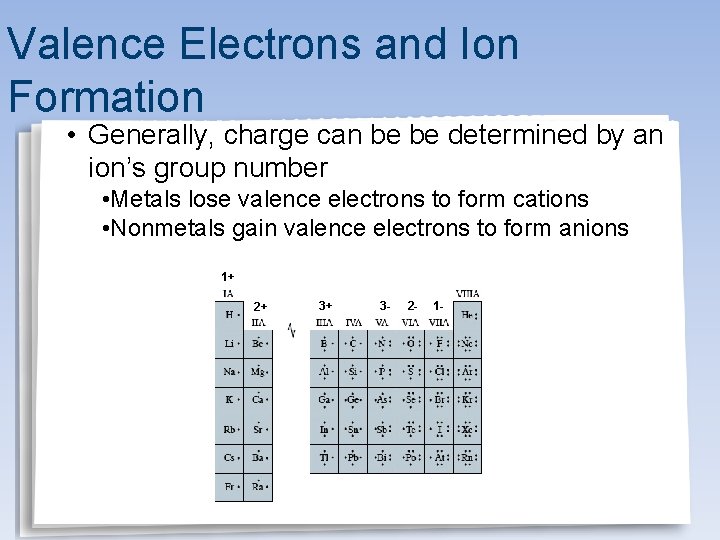
Valence Electrons and Ion Formation • Generally, charge can be be determined by an ion’s group number • Metals lose valence electrons to form cations • Nonmetals gain valence electrons to form anions 1+ 2+ 3+ 3 - 2 - 1 -

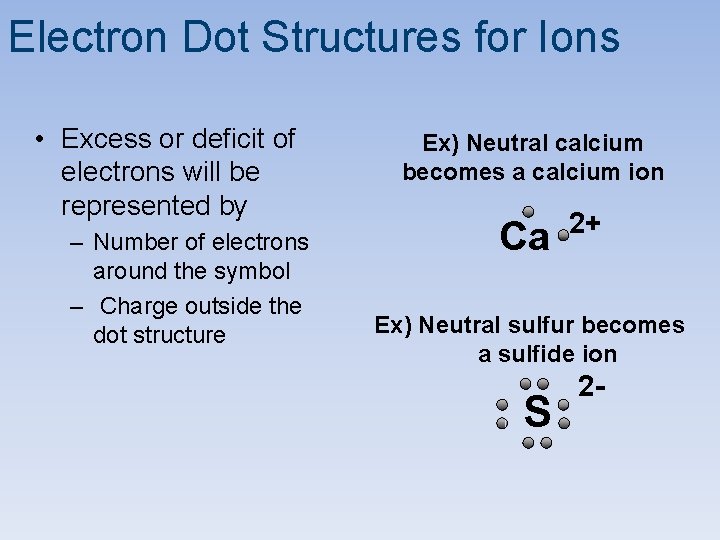
Electron Dot Structures for Ions • Excess or deficit of electrons will be represented by – Number of electrons around the symbol – Charge outside the dot structure Ex) Neutral calcium becomes a calcium ion Ca 2+ Ex) Neutral sulfur becomes a sulfide ion S 2 -

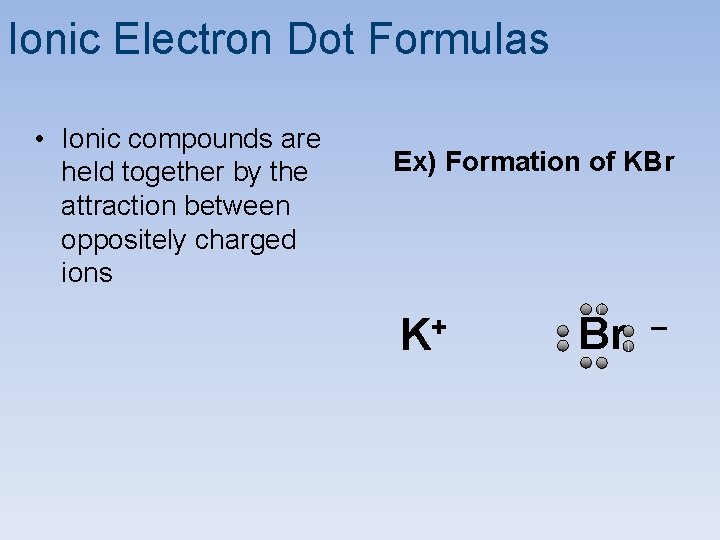
Ionic Electron Dot Formulas • Ionic compounds are held together by the attraction between oppositely charged ions Ex) Formation of KBr K+ Br –

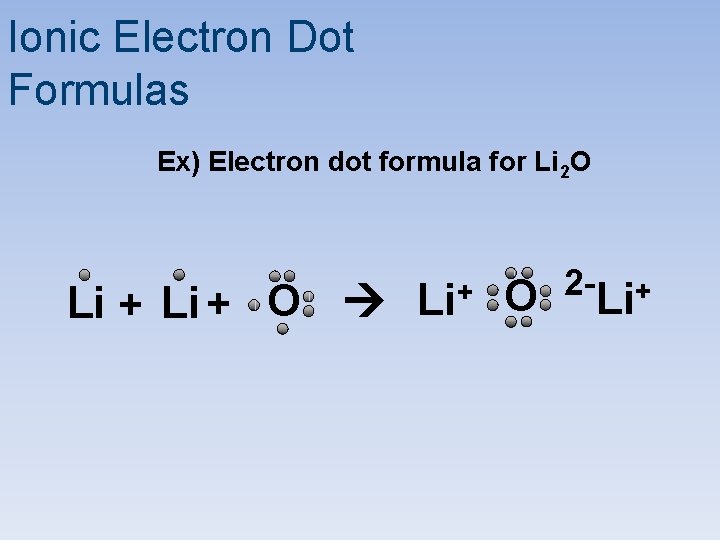
Ionic Electron Dot Formulas Ex) Electron dot formula for Li 2 O Li + O Li+ O 2 - Li+

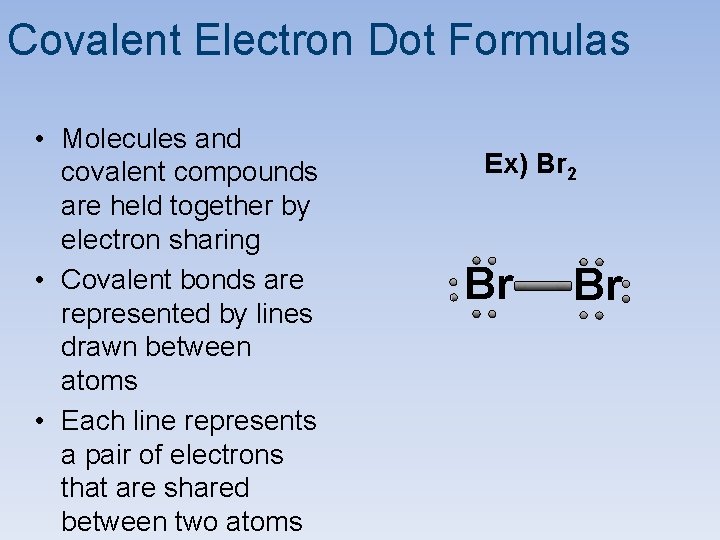
Covalent Electron Dot Formulas • Molecules and covalent compounds are held together by electron sharing • Covalent bonds are represented by lines drawn between atoms • Each line represents a pair of electrons that are shared between two atoms Ex) Br 2 Br Br

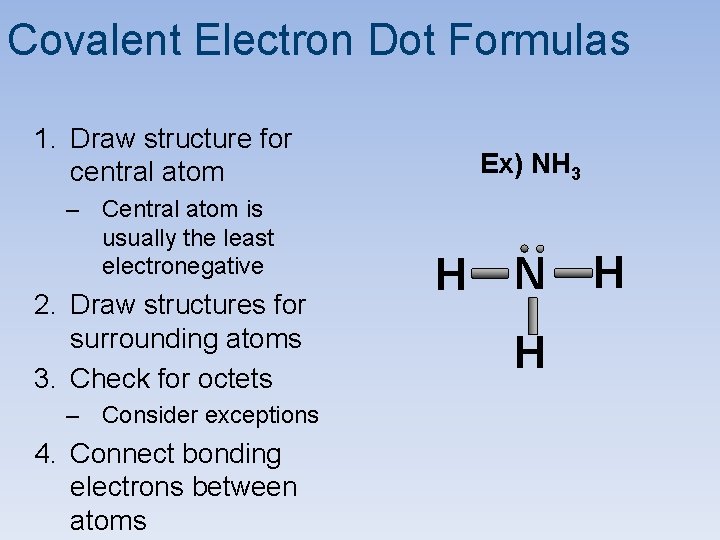
Covalent Electron Dot Formulas 1. Draw structure for central atom – Central atom is usually the least electronegative 2. Draw structures for surrounding atoms 3. Check for octets – Consider exceptions 4. Connect bonding electrons between atoms Ex) NH 3 H N H H

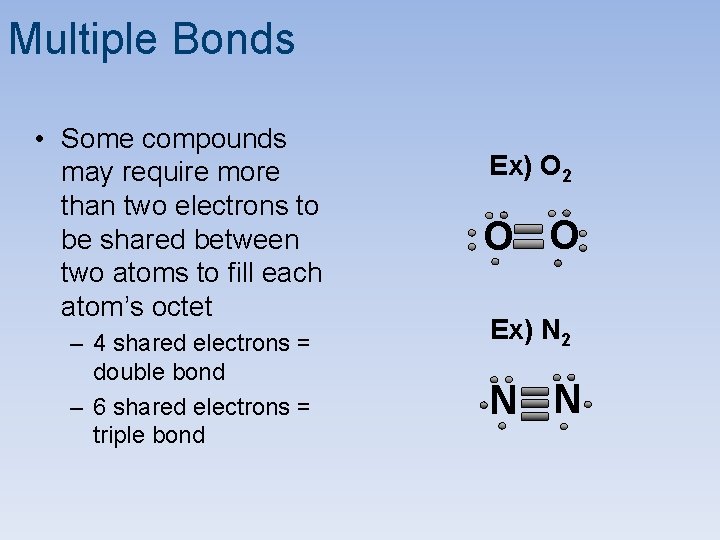
Multiple Bonds • Some compounds may require more than two electrons to be shared between two atoms to fill each atom’s octet – 4 shared electrons = double bond – 6 shared electrons = triple bond Ex) O 2 O O Ex) N 2 N N

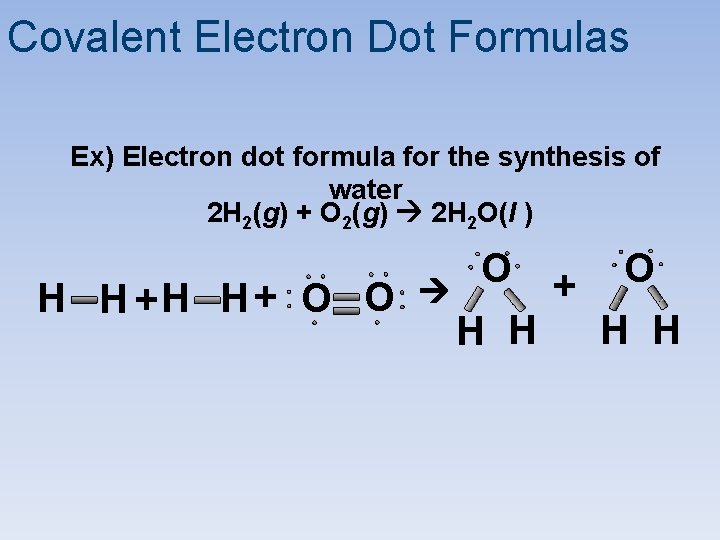
Covalent Electron Dot Formulas Ex) Electron dot formula for the synthesis of water 2 H 2(g) + O 2(g) 2 H 2 O(l ) H H + O O O H H + O H H

Electron Dot Formulas Lesson Objectives • Draw electron dot formulas – Ionic compounds – Covalent compounds