Electron Dot Diagrams for Ionic Compounds 1 Using


- Slides: 14


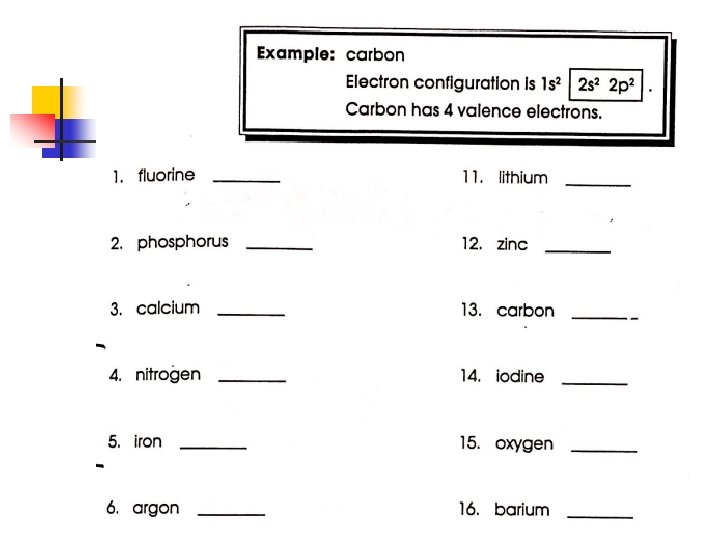
Electron Dot Diagrams for Ionic Compounds 1. Using the octet rule, describe the formation of a cation or an anion from an element 2. Create electron dot diagrams for ionic compounds.

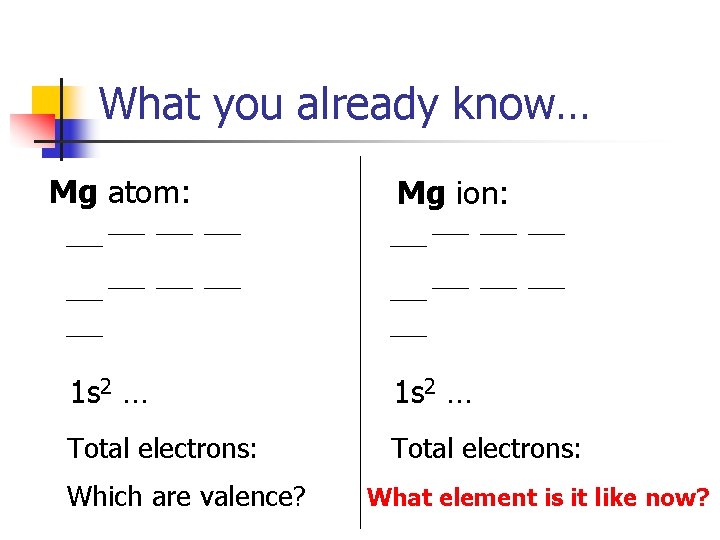
What you already know… Mg atom: Mg ion: 1 s 2 … Total electrons: Which are valence? What element is it like now?

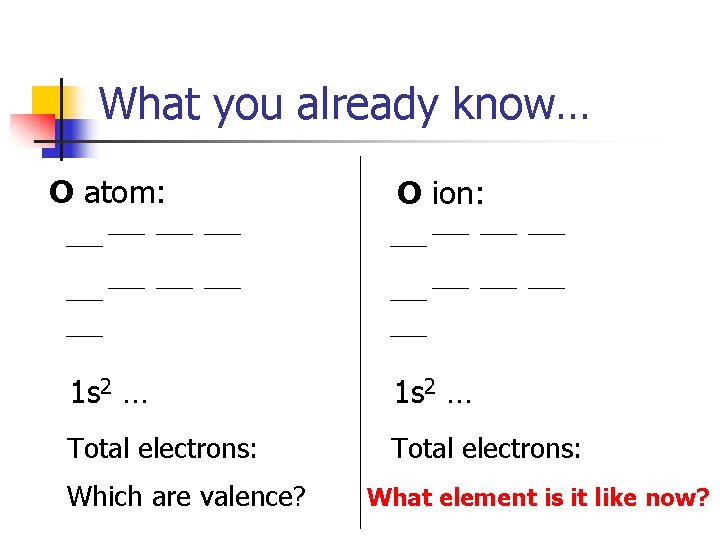
What you already know… O atom: O ion: 1 s 2 … Total electrons: Which are valence? What element is it like now?

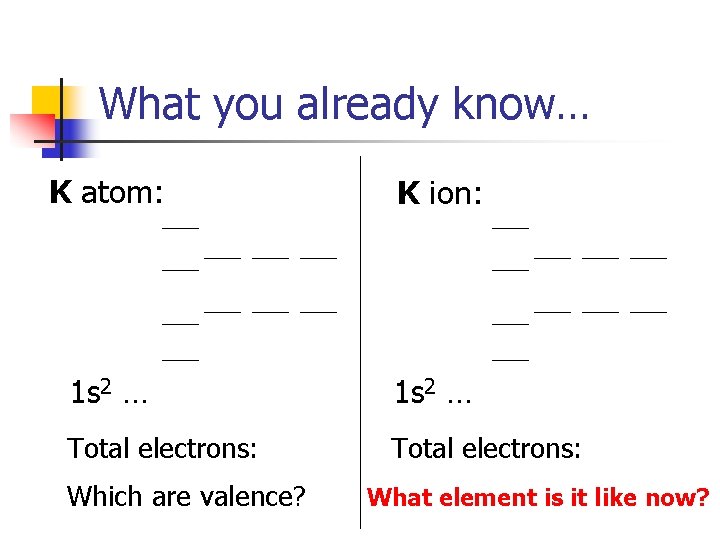
What you already know… K atom: K ion: 1 s 2 … Total electrons: Which are valence? What element is it like now?

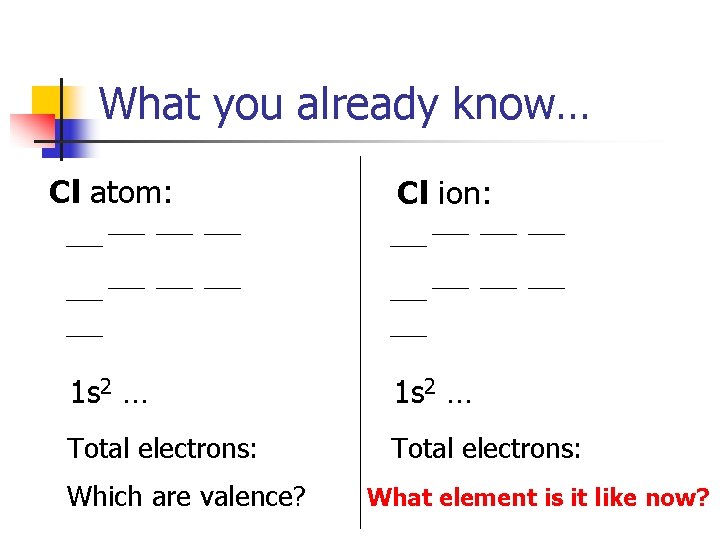
What you already know… Cl atom: Cl ion: 1 s 2 … Total electrons: Which are valence? What element is it like now?

What you don’t know… Where do the electrons go to when they are lost? ? ? Where do the electrons come from when they gained? ? ?

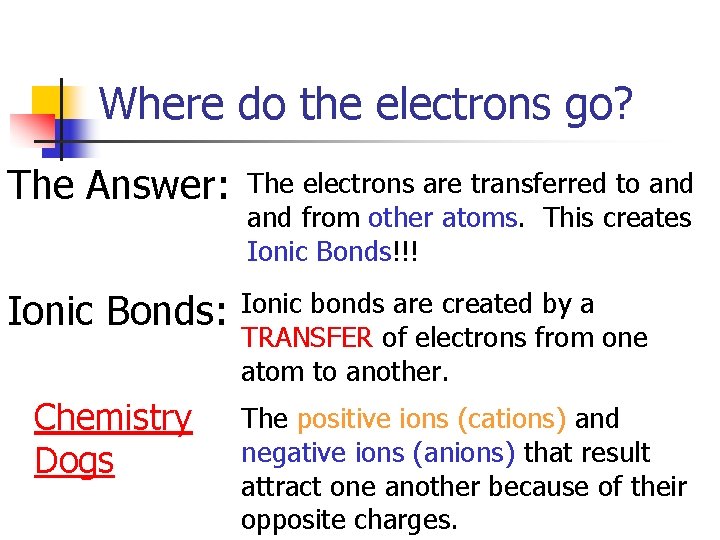
Where do the electrons go? The Answer: The electrons are transferred to and from other atoms. This creates Ionic Bonds!!! Ionic Bonds: Ionic bonds are created by a TRANSFER of electrons from one atom to another. Chemistry Dogs The positive ions (cations) and negative ions (anions) that result attract one another because of their opposite charges.

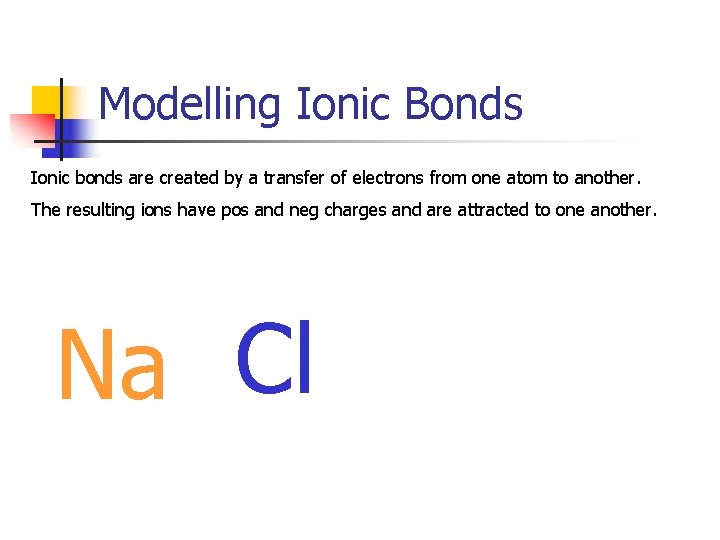
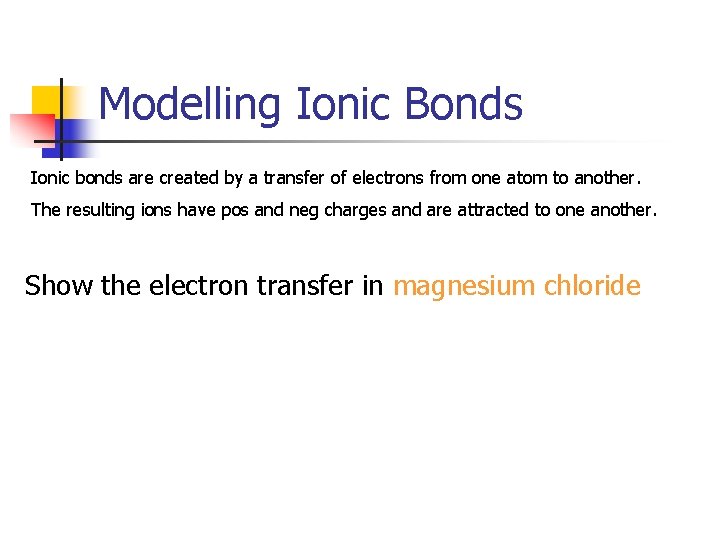
Modelling Ionic Bonds Ionic bonds are created by a transfer of electrons from one atom to another. The resulting ions have pos and neg charges and are attracted to one another. Na Cl

Modelling Ionic Bonds Ionic bonds are created by a transfer of electrons from one atom to another. The resulting ions have pos and neg charges and are attracted to one another. Show the electron transfer in magnesium chloride

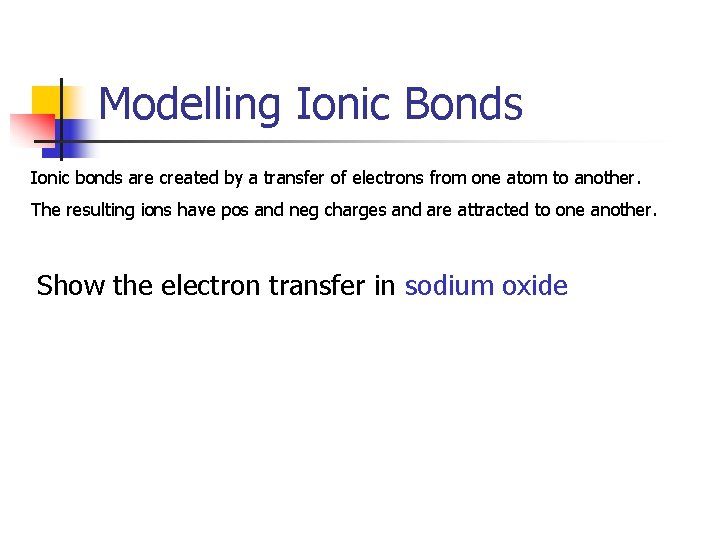
Modelling Ionic Bonds Ionic bonds are created by a transfer of electrons from one atom to another. The resulting ions have pos and neg charges and are attracted to one another. Show the electron transfer in sodium oxide

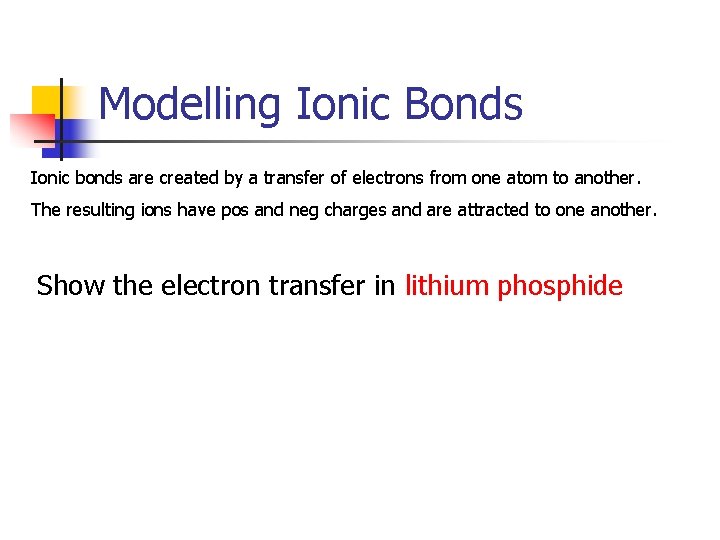
Modelling Ionic Bonds Ionic bonds are created by a transfer of electrons from one atom to another. The resulting ions have pos and neg charges and are attracted to one another. Show the electron transfer in lithium phosphide

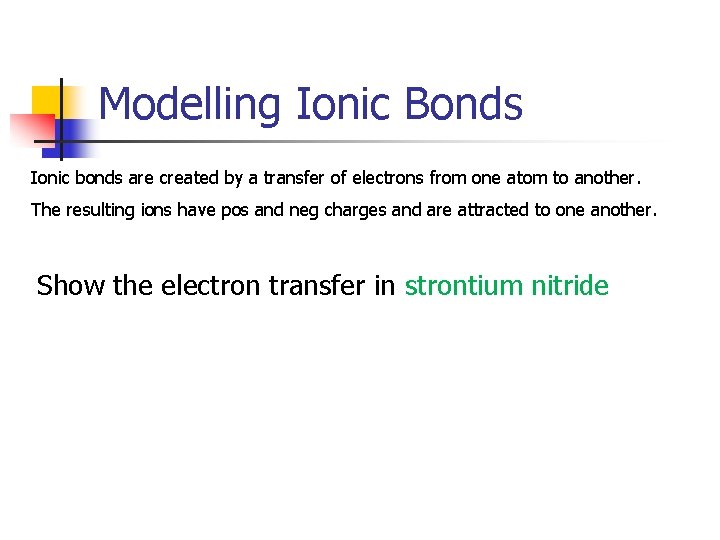
Modelling Ionic Bonds Ionic bonds are created by a transfer of electrons from one atom to another. The resulting ions have pos and neg charges and are attracted to one another. Show the electron transfer in strontium nitride

Practice on your own: Draw arrows to show the electron transfer in the following ionic compounds: 1. Calcium chloride 2. Magnesium oxide