Electron Dot Diagrams and Ions Electron Dot Diagrams




- Slides: 14

Electron Dot Diagrams and Ions

Electron Dot Diagrams l l 1. 2. 3. Show ONLY outer level electrons, or valence e. Also called Lewis diagrams Begin with the element’s symbol Use the PT to determine the number of outer level electrons Place up to 2 dots per side for a total of up to 8 electrons

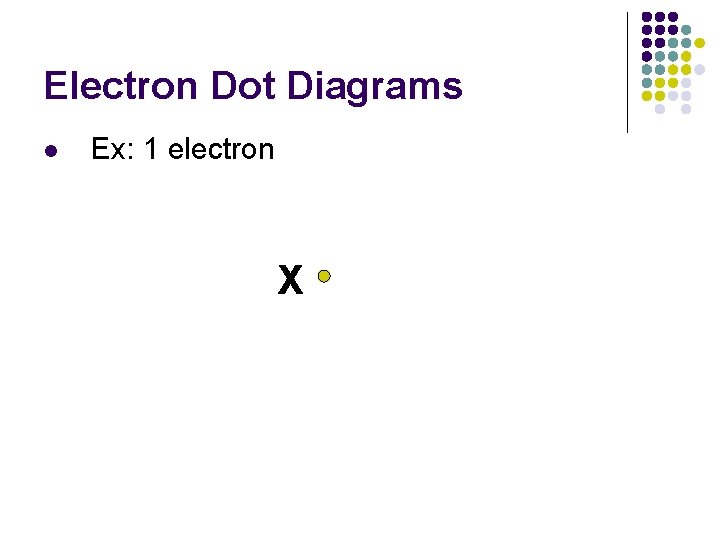
Electron Dot Diagrams l Ex: 1 electron X

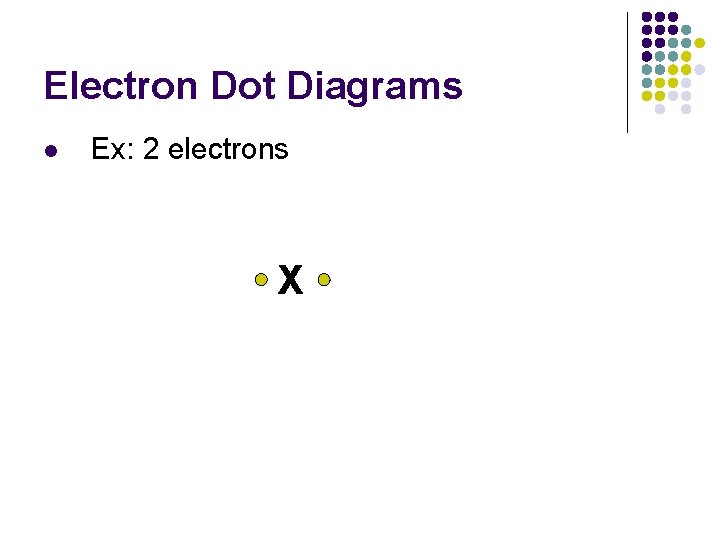
Electron Dot Diagrams l Ex: 2 electrons X

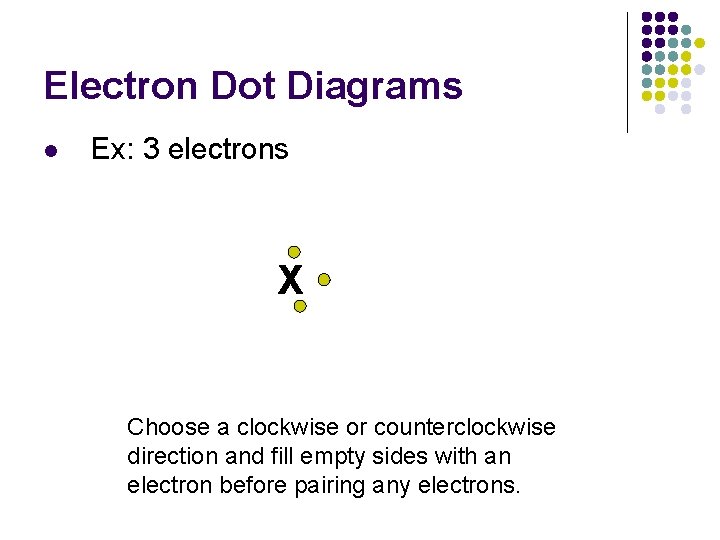
Electron Dot Diagrams l Ex: 3 electrons X Choose a clockwise or counterclockwise direction and fill empty sides with an electron before pairing any electrons.

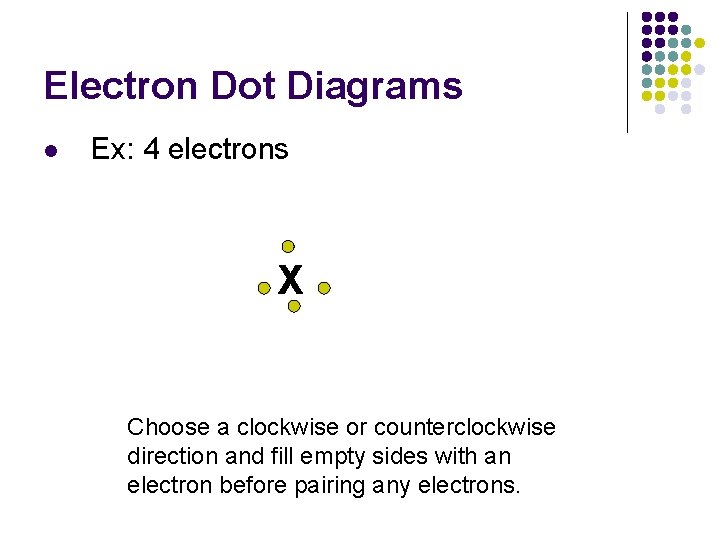
Electron Dot Diagrams l Ex: 4 electrons X Choose a clockwise or counterclockwise direction and fill empty sides with an electron before pairing any electrons.

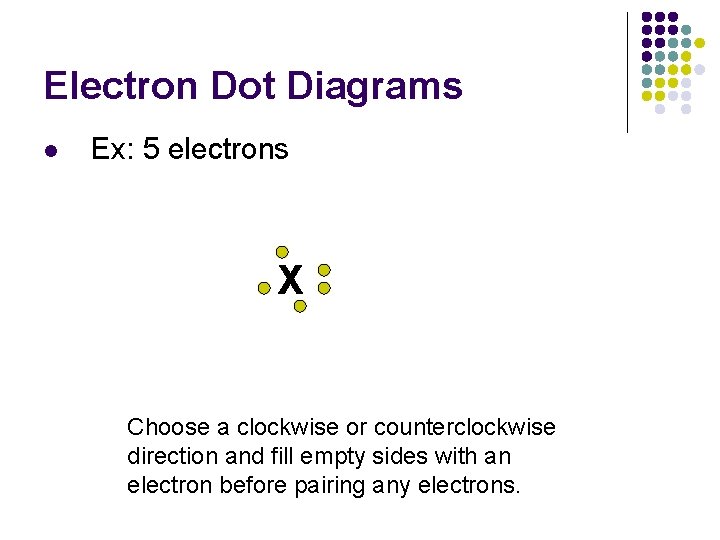
Electron Dot Diagrams l Ex: 5 electrons X Choose a clockwise or counterclockwise direction and fill empty sides with an electron before pairing any electrons.

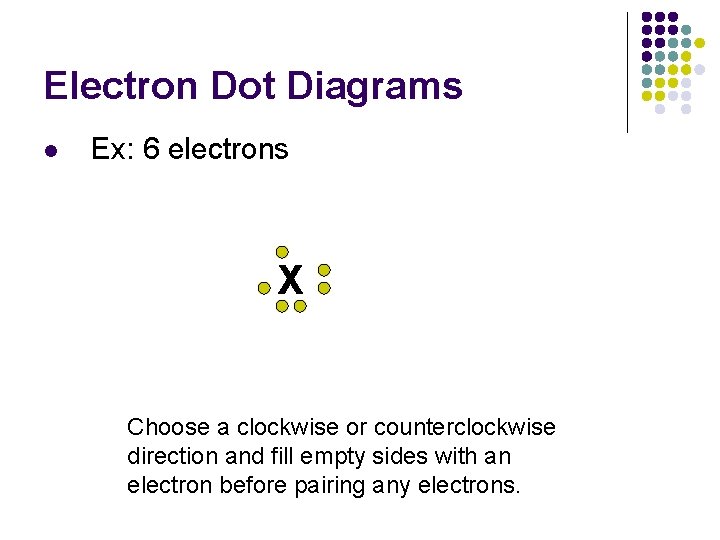
Electron Dot Diagrams l Ex: 6 electrons X Choose a clockwise or counterclockwise direction and fill empty sides with an electron before pairing any electrons.

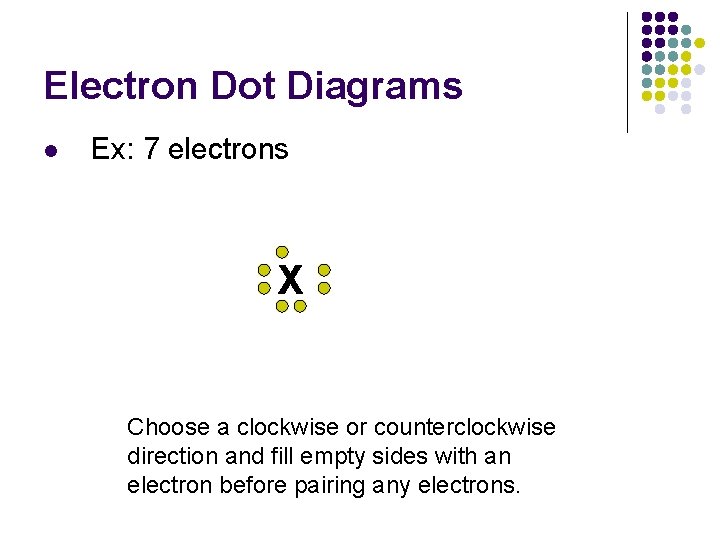
Electron Dot Diagrams l Ex: 7 electrons X Choose a clockwise or counterclockwise direction and fill empty sides with an electron before pairing any electrons.

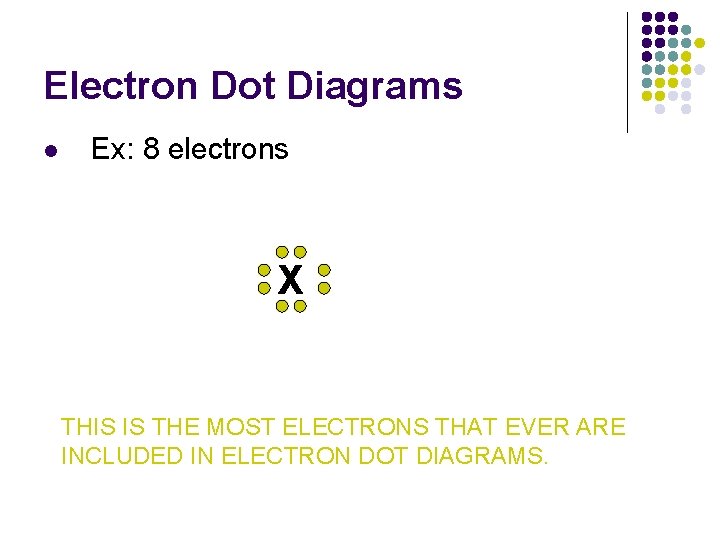
Electron Dot Diagrams l Ex: 8 electrons X THIS IS THE MOST ELECTRONS THAT EVER ARE INCLUDED IN ELECTRON DOT DIAGRAMS.

Electron Dot Diagrams l l Time to practice what you’ve learned For the following elements, create electron dot diagrams. aluminum potassium argon sulfur phosphorus silicon barium iodine

CHEMICAL BONDING The number of electrons in the _______energy level determines whether an atom will form bonds. l l Atoms bond to get a ____ outer level. l l These electrons are also called ______ electrons. For all E levels beyond the first, the outermost E level is considered to be full if it contains ______ (#) electrons. The first E level is full with ____ (#) electrons. Why are noble gases nonreactive?

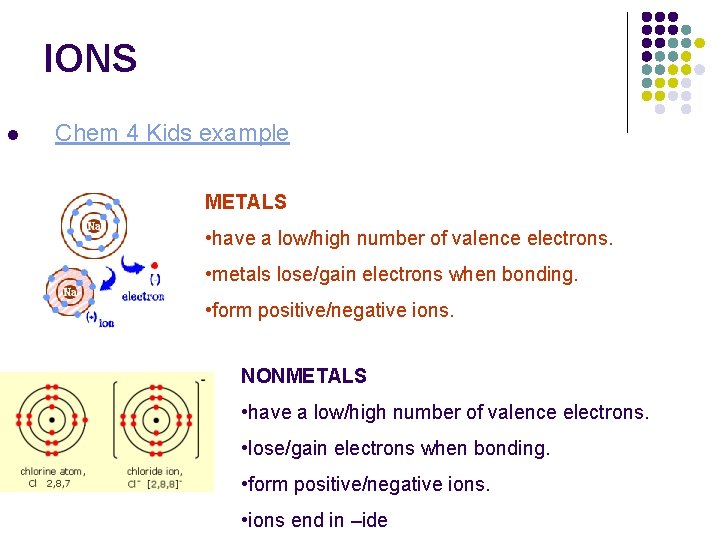
IONS l Chem 4 Kids example METALS • have a low/high number of valence electrons. • metals lose/gain electrons when bonding. • form positive/negative ions. NONMETALS • have a low/high number of valence electrons. • lose/gain electrons when bonding. • form positive/negative ions. • ions end in –ide

IONS- Practice l l l l aluminum potassium argon oxygen phosphorus silicon barium iodine l Write the ions formed by the elements.