Electron Distribution Goal Determine electron structures in atoms

Electron Distribution • Goal: Determine electron structures in atoms • Orbital level • Sublevel
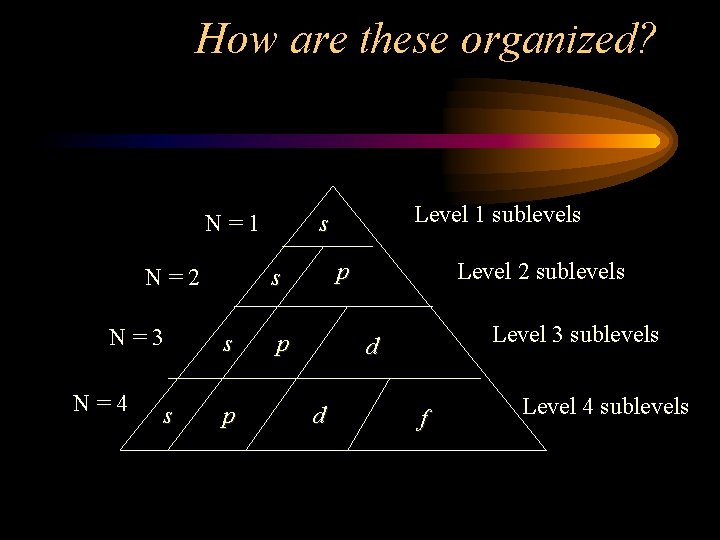
How are these organized? N=1 N=2 N=3 N=4 s p s s p Level 1 sublevels s p Level 2 sublevels Level 3 sublevels d d f Level 4 sublevels
Remember: 1 s is smaller than 2 s, 2 s is smaller than 3 s, etc. How about energy? Which has the most?

How do atoms put their electrons in these sublevels? • Lowest energy levels are filled first. • Sublevels are filled “s”, then “p”, then “d”, then “f”. • Well, sort of. . .
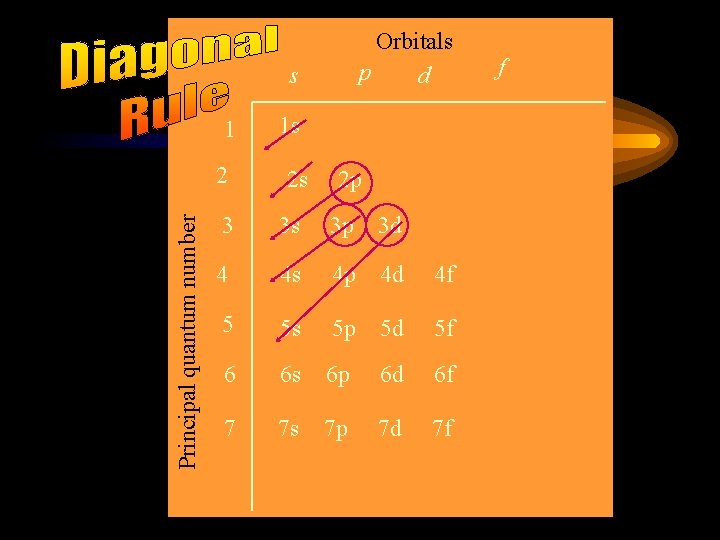
Orbitals p d s 1 Principal quantum number 2 1 s 2 s 2 p 3 3 s 3 p 3 d 4 4 s 4 p 4 d 4 f 5 5 s 5 p 5 d 5 f 6 6 s 6 p 6 d 6 f 7 7 s 7 p 7 d 7 f f
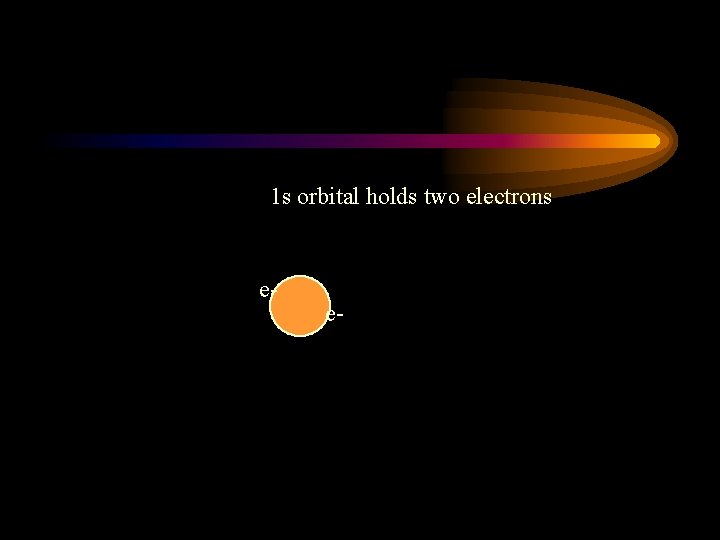
1 s orbital holds two electrons e- e-
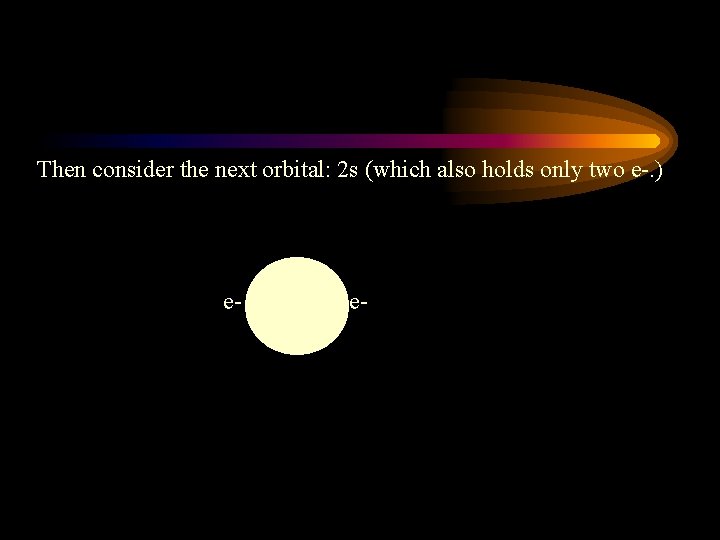
Then consider the next orbital: 2 s (which also holds only two e-. ) e- e-
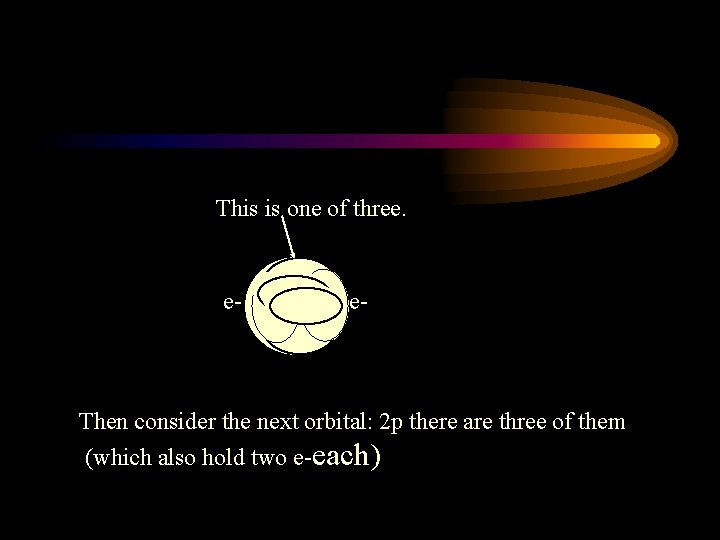
This is one of three. e- e- Then consider the next orbital: 2 p there are three of them (which also hold two e-each)

Electron configuration for … Scandium • Look up the number of electrons (same as the number of protons). • Use the diagonal rule. 26 21 • Scandium is #21. That means Sc has _____ electrons.
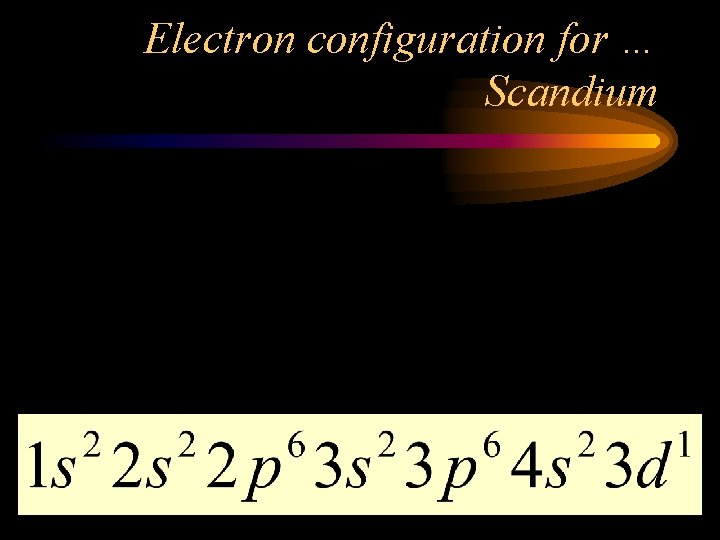
Electron configuration for … Scandium
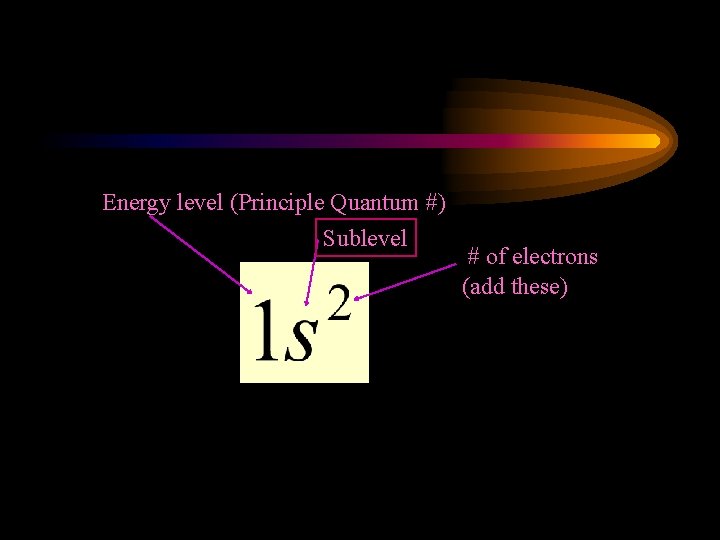
Energy level (Principle Quantum #) Sublevel # of electrons (add these)
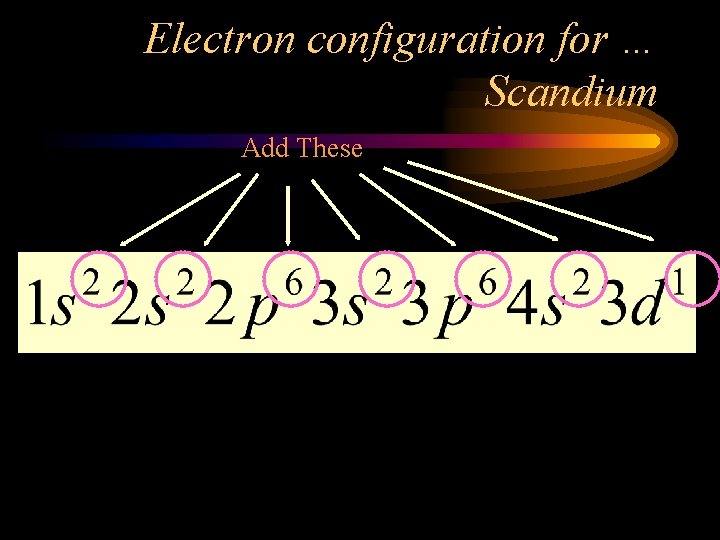
Electron configuration for … Scandium Add These
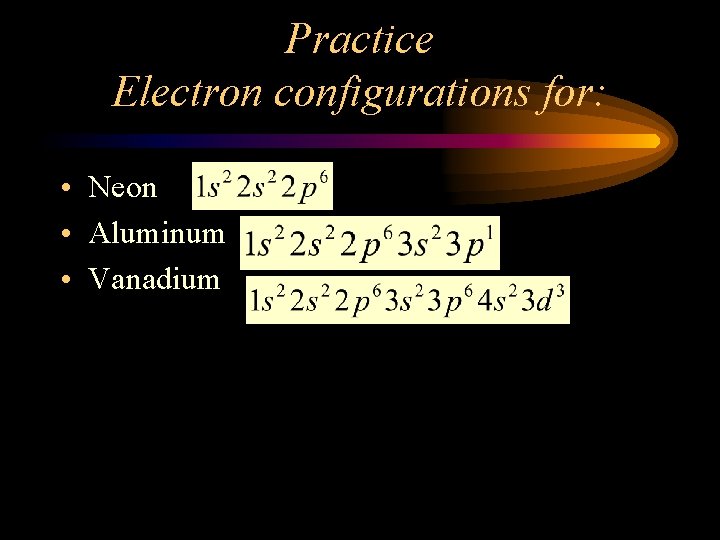
Practice Electron configurations for: • Neon • Aluminum • Vanadium
- Slides: 13