Electron Configurations You will learn how to write



- Slides: 54

Electron Configurations. You will learn how to write electron configurations, and how these electron arrangements relate to the shape and lay-out of the periodic table. Because electrons are so important in chemistry, the way in which they are arranged around the nucleus plays a crucial role in determining the chemical reactivity of all the elements.

First let’s review a little: As we have learned, electrons exist in very specific energy levels. And when these electrons absorb energy… They get energized up to higher levels. Actually, the jump to higher levels is not a gradual transition as was just shown. It is a “quantum” jump, and looks more like this: Quantum means it happens all at once – instantaneously – because the electron can never exist between levels – not even for a second.

Once it is at this higher level (excited state), it doesn’t stay there long. It quickly drops down to a lower level – again as a quantum leap – and as it does, it gives off a distinct band of light energy. Also, notice how the electron doesn’t have to drop all the way back down to the lowest level. It can get energized up to any level, and from there it can drop to any lower level. AND the different drops each produce different frequencies of light. And a 52 drop produces violet light See how an 2 electron dropping from the A 4 electron produces blue light 3 rd level to the 2 nd level produced red light

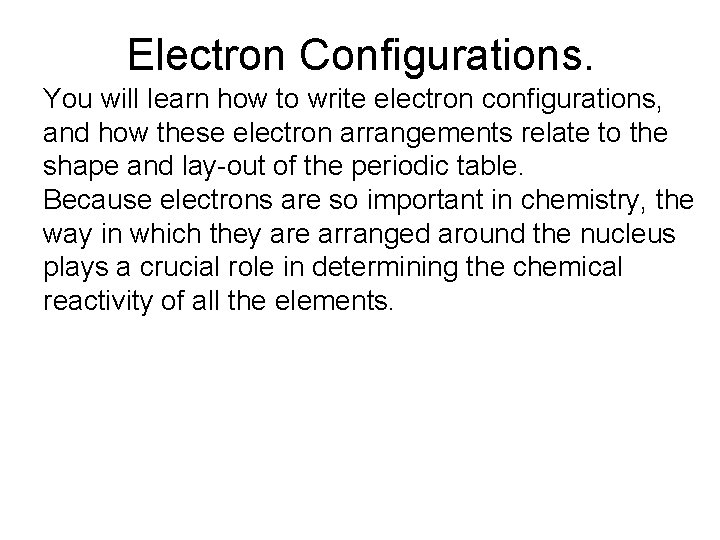
7 th 6 th 5 th 4 th 3 rd 2 nd Now let’s take a closer look at these electron energy levels. We’ll color code them to make them easier to distinguish. . . We will see in a moment that these levels are actually made up of sublevels. The higher up you go, the more sublevels there are. We will represent these sublevels as lines with boxes on them. The boxes represent the “orbitals” that make up the sublevels. This is where the electrons hang out. A maximum of two electrons can fit in any orbital. The sublevel shown below is part of the 2 nd level and it is called the “ 2 p sublevel. ” Note that the 2 p sublevel is made up of 3 orbitals (see three boxes). And since two electrons can fit in each orbital, the 2 p sublevel is capable of holding a maximum of six electrons: 2 p Also, let’s not forget about the nucleus… It is positively charged (because of all the protons in it). 1 st After all, it is this positively charged nucleus that holds the negatively charged electrons around it in the first place.

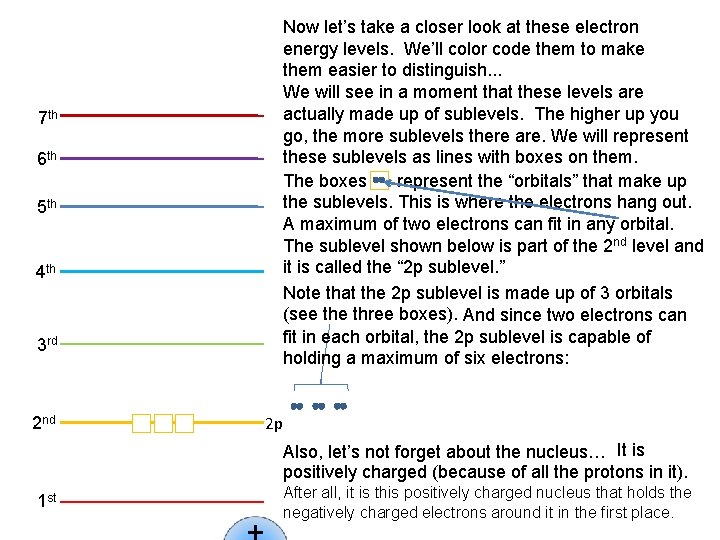
And with the 6 th and 7 th, it just gets worse and worse! 7 th 7 s 7 g 7 h 7 i 7 f 7 d 7 p 6 th 6 g 6 h 6 f 6 d 6 p 6 s 5 th 5 g 5 f 5 s 5 d 5 p 4 th 4 d 4 f 4 s 4 p 3 rd 3 p 3 d 3 s 2 nd 2 p 2 s 1 st 1 s The 5 th level is just what you would expect: Five sublevels: 5 s (1), 5 p (3), 5 d (5), 5 f (7) and 5 g (9) But look at how extensive the overlap becomes. How about the 4 th level? You should have a pretty good idea about everything concerning it, accept what the new letter is. Is this what you were thinking? 4 s (1), 4 p (3), 4 d (5), and 4 f (7). See how the number of orbitals is always a consecutive odd number? Also notice how the sublevels start to overlap. The 4 s is actually a little lower than the 3 d. This overlap is very important, and it becomes more extensive as we move to higher levels. Any guesses about the 3 rd level? The 3 rd is made up of 3 sublevels [Do you see the pattern? ] The 3 s sublevel (1 orbital), the 3 p sublevel (3 orbitals) and the 3 d sublevel (5 orbitals) The 2 nd level is made up of 2 sublevels: The 2 s sublevel (which, like the 1 s, contains just one orbital), and the 2 p sublevel which contains three orbitals. So let’s start at the bottom. The 1 st energy level is comprised of just one sublevel: It is called the 1 s sublevel, and it contains just one orbital. That’s it!

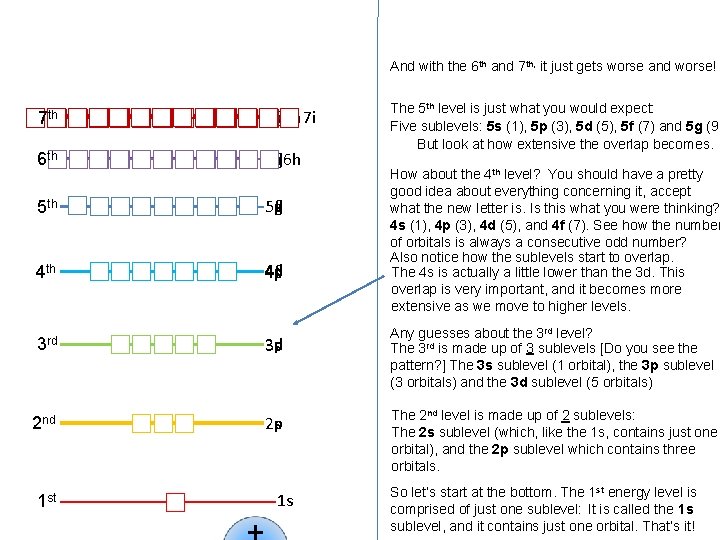
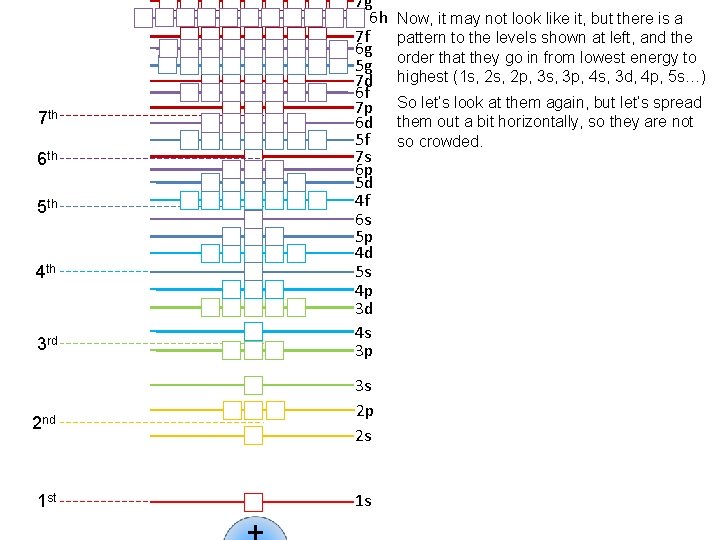
7 th 6 th 5 th 4 th 3 rd 2 nd 1 st 7 g 6 h 7 f 6 g 5 g 7 d 6 f 7 p 6 d 5 f 7 s 6 p 5 d 4 f 6 s 5 p 4 d 5 s 4 p 3 d 4 s 3 p 3 s 2 p 2 s 1 s Now, it may not look like it, but there is a pattern to the levels shown at left, and the order that they go in from lowest energy to highest (1 s, 2 p, 3 s, 3 p, 4 s, 3 d, 4 p, 5 s…) So let’s look at them again, but let’s spread them out a bit horizontally, so they are not so crowded.

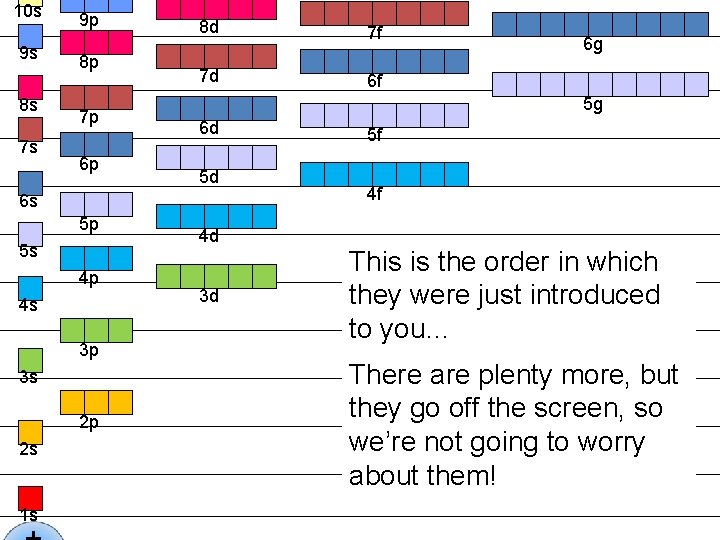
10 s 9 s 8 s 7 s 9 p 8 p 7 p 6 p 8 d 7 f 7 d 6 f 5 g 6 d 5 d 6 s 5 p 5 s 4 p 4 s 3 p 3 s 2 p 2 s 1 s 6 g 4 d 3 d 5 f 4 f This is the order in which they were just introduced to you… There are plenty more, but they go off the screen, so we’re not going to worry about them!

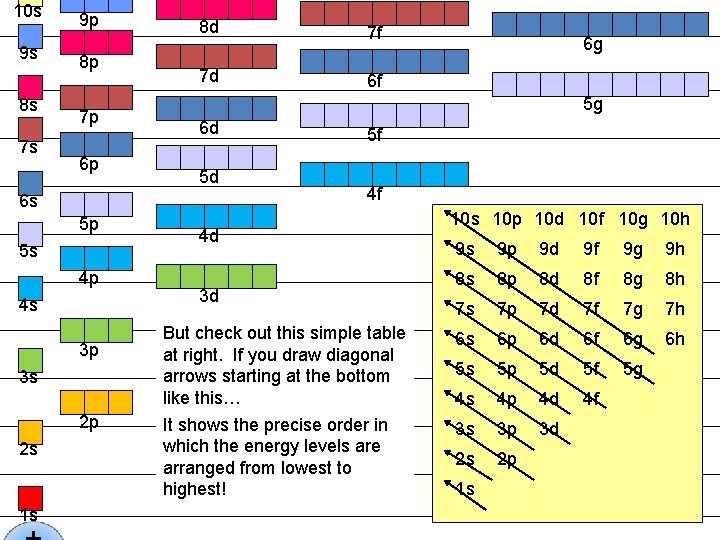
10 s 9 s 8 s 7 s 9 p 8 p 7 p 6 p 8 d 7 f 7 d 6 f 5 g 6 d 5 d 6 s 5 p 5 s 4 p 4 s 3 p 3 s 2 p 2 s 1 s 6 g 5 f 4 f 4 d 3 d But check out this simple table at right. If you draw diagonal arrows starting at the bottom like this… It shows the precise order in which the energy levels are arranged from lowest to highest! 10 s 10 p 10 d 10 f 10 g 10 h 9 s 9 p 9 d 9 f 9 g 9 h 8 s 8 p 8 d 8 f 8 g 8 h 7 s 7 p 7 d 7 f 7 g 7 h 6 s 6 p 6 d 6 f 6 g 6 h 5 s 5 p 5 d 5 f 5 g 4 s 4 p 4 d 4 f 3 s 3 p 3 d 2 s 2 p 1 s

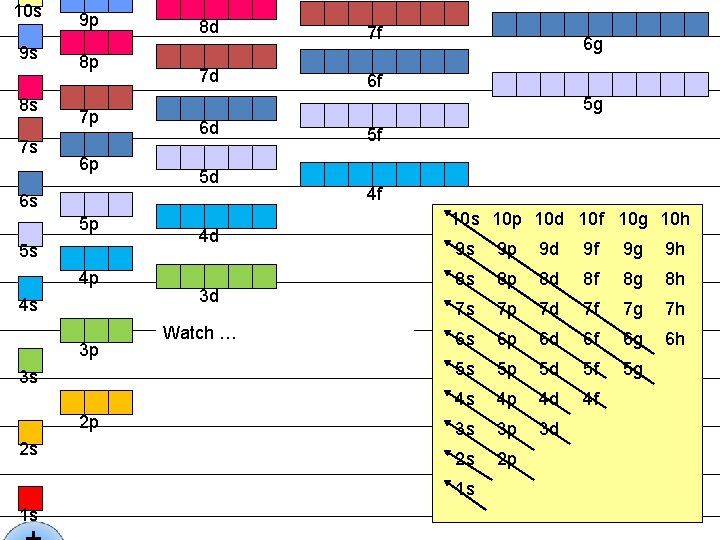
10 s 9 s 8 s 7 s 9 p 8 p 7 p 6 p 8 d 7 f 7 d 6 f 5 g 6 d 5 d 6 s 5 p 5 s 4 p 4 s 3 p 3 s 2 p 2 s 6 g 4 d 3 d Watch … 5 f 4 f 10 s 10 p 10 d 10 f 10 g 10 h 9 s 9 p 9 d 9 f 9 g 9 h 8 s 8 p 8 d 8 f 8 g 8 h 7 s 7 p 7 d 7 f 7 g 7 h 6 s 6 p 6 d 6 f 6 g 6 h 5 s 5 p 5 d 5 f 5 g 4 s 4 p 4 d 4 f 3 s 3 p 3 d 2 s 2 p 1 s 1 s

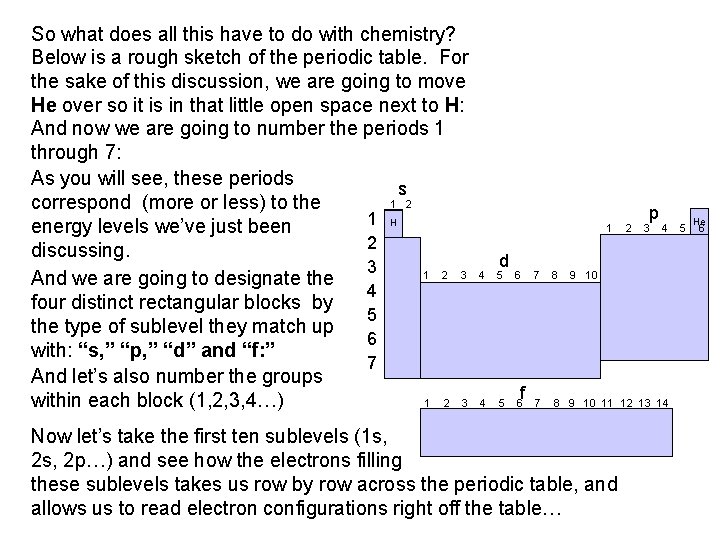
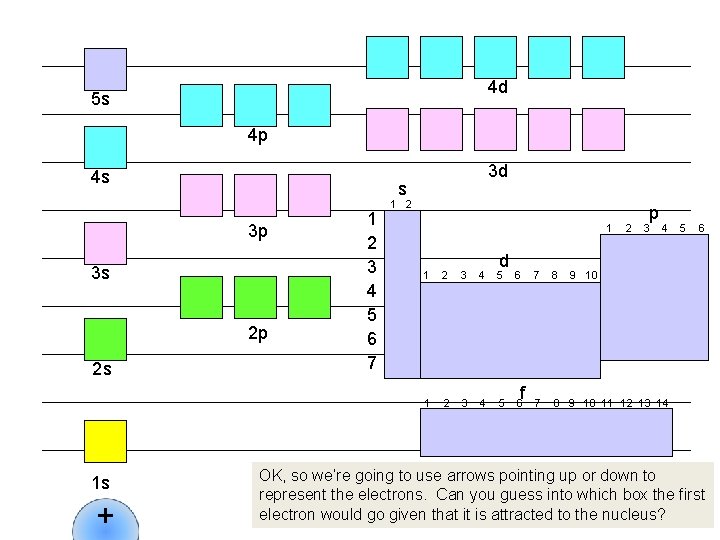
So what does all this have to do with chemistry? Below is a rough sketch of the periodic table. For the sake of this discussion, we are going to move He over so it is in that little open space next to H: And now we are going to number the periods 1 through 7: As you will see, these periods s 1 2 correspond (more or less) to the 1 H energy levels we’ve just been 2 discussing. 3 1 2 3 And we are going to designate the 4 four distinct rectangular blocks by 5 the type of sublevel they match up 6 with: “s, ” “p, ” “d” and “f: ” 7 And let’s also number the groups 1 2 3 within each block (1, 2, 3, 4…) 1 d 4 5 6 f 2 p 3 4 7 8 9 10 11 12 13 14 Now let’s take the first ten sublevels (1 s, 2 p…) and see how the electrons filling these sublevels takes us row by row across the periodic table, and allows us to read electron configurations right off the table… 5 He 6

4 d 5 s 4 p 4 s s 3 p 3 s 2 p 2 s 1 s + 3 d 1 2 3 4 5 6 7 1 2 1 d 1 2 3 4 5 6 f 2 p 3 4 7 8 9 10 11 12 13 14 5 6 9 10 OK, so we’re going to use arrows pointing up or down to represent the electrons. Can you guess into which box the first electron would go given that it is attracted to the nucleus?

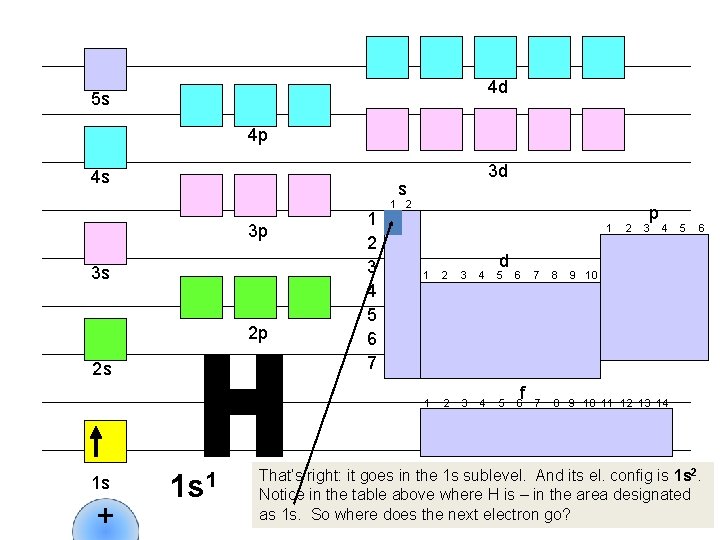
4 d 5 s 4 p 4 s s 3 p 3 s 2 p 2 s 1 s + 3 d 1 s 1 1 2 3 4 5 6 7 1 2 1 d 1 2 3 4 5 6 f 2 p 3 4 7 8 9 10 11 12 13 14 5 6 9 10 That’s right: it goes in the 1 s sublevel. And its el. config is 1 s 2. Notice in the table above where H is – in the area designated as 1 s. So where does the next electron go?

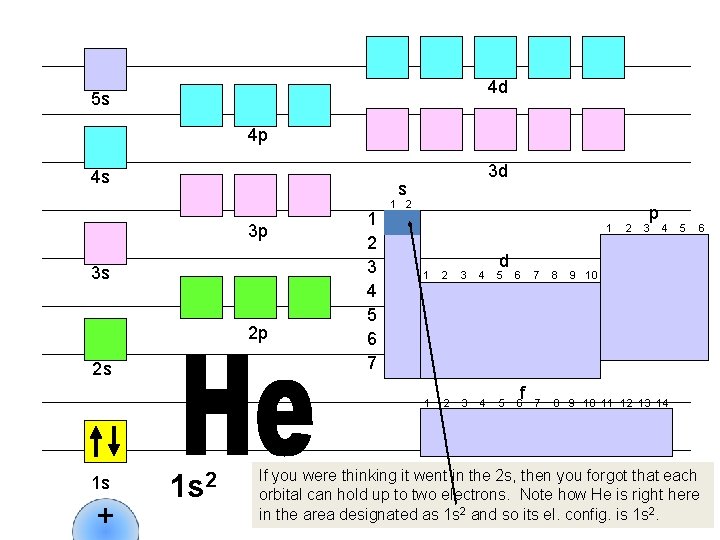
4 d 5 s 4 p 4 s s 3 p 3 s 2 p 2 s 1 s + 3 d 1 s 2 1 2 3 4 5 6 7 1 2 1 d 1 2 3 4 5 6 f 2 p 3 4 7 8 9 10 11 12 13 14 5 6 9 10 If you were thinking it went in the 2 s, then you forgot that each orbital can hold up to two electrons. Note how He is right here in the area designated as 1 s 2 and so its el. config. is 1 s 2.

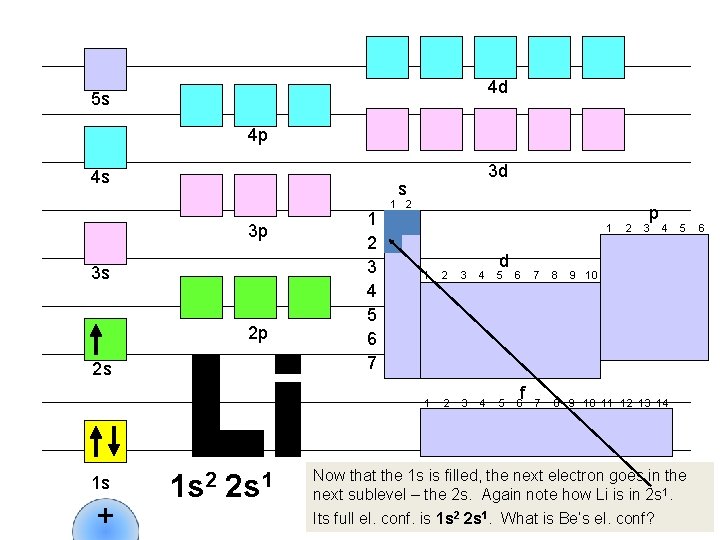
4 d 5 s 4 p 4 s s 3 p 3 s 2 p 2 s 1 s + 3 d 1 s 2 2 s 1 1 2 3 4 5 6 7 1 2 1 d 1 2 3 4 5 6 f 2 p 3 4 7 8 9 10 11 12 13 14 5 9 10 Now that the 1 s is filled, the next electron goes in the next sublevel – the 2 s. Again note how Li is in 2 s 1. Its full el. conf. is 1 s 2 2 s 1. What is Be’s el. conf? 6

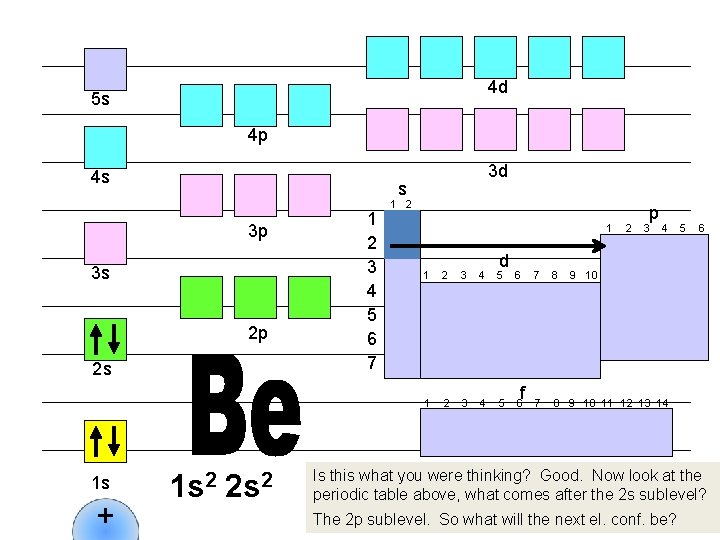
4 d 5 s 4 p 4 s s 3 p 3 s 2 p 2 s 1 s + 3 d 1 s 2 2 s 2 1 2 3 4 5 6 7 1 2 1 d 1 2 3 4 5 6 f 2 p 3 4 7 8 9 10 11 12 13 14 5 6 9 10 Is this what you were thinking? Good. Now look at the periodic table above, what comes after the 2 s sublevel? The 2 p sublevel. So what will the next el. conf. be?

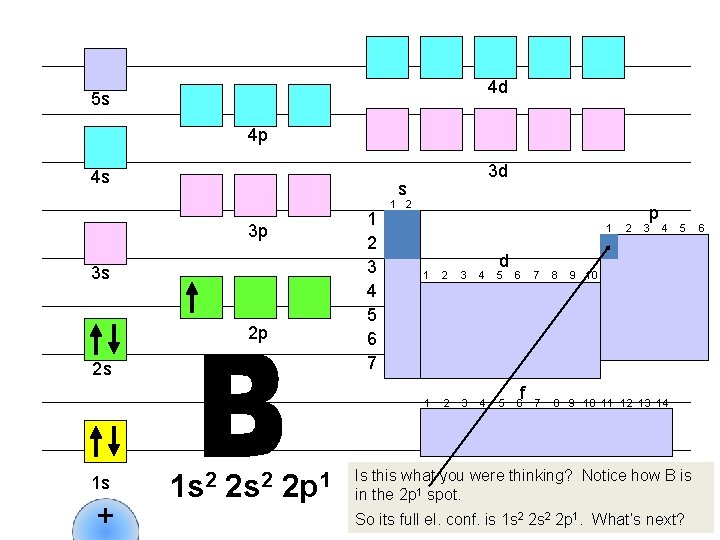
4 d 5 s 4 p 4 s s 1 2 3 4 5 6 7 3 p 3 s 2 p 2 s 1 s + 3 d 1 s 2 2 p 1 1 2 1 d 1 2 3 4 5 6 f 2 p 3 4 7 8 9 10 11 12 13 14 5 9 10 Is this what you were thinking? Notice how B is in the 2 p 1 spot. So its full el. conf. is 1 s 2 2 p 1. What’s next? 6

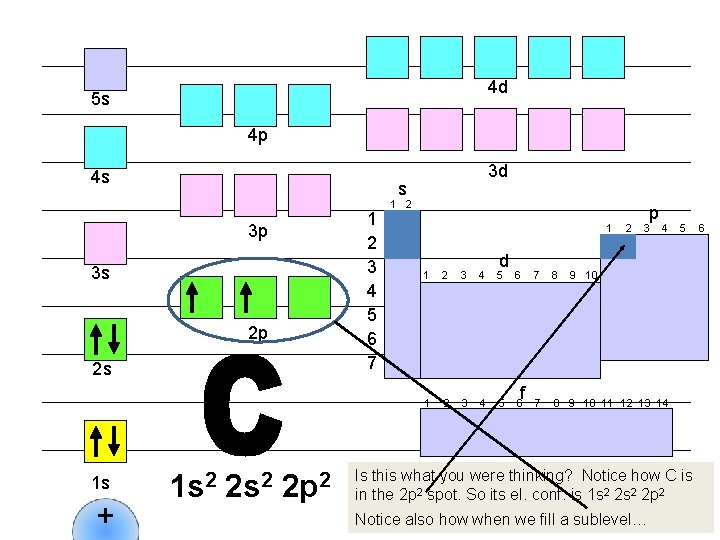
4 d 5 s 4 p 4 s s 1 2 3 4 5 6 7 3 p 3 s 2 p 2 s 1 s + 3 d 1 s 2 2 p 2 1 d 1 2 3 4 5 6 f 2 p 3 4 7 8 9 10 11 12 13 14 5 9 10 Is this what you were thinking? Notice how C is in the 2 p 2 spot. So its el. conf. is 1 s 2 2 p 2 Notice also how when we fill a sublevel… 6

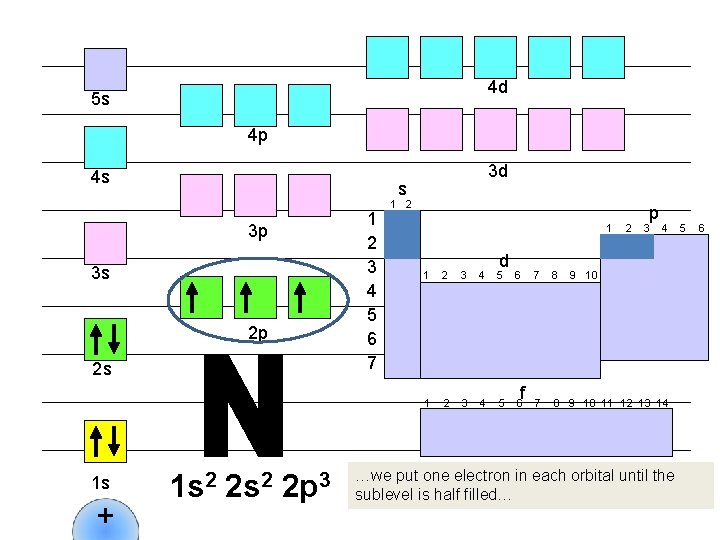
4 d 5 s 4 p 4 s s 1 2 3 4 5 6 7 3 p 3 s 2 p 2 s 1 s + 3 d 1 s 2 2 p 3 1 2 1 d 1 2 3 4 5 6 f 2 p 3 4 7 8 9 10 11 12 13 14 …we put one electron in each orbital until the sublevel is half filled… 5 6

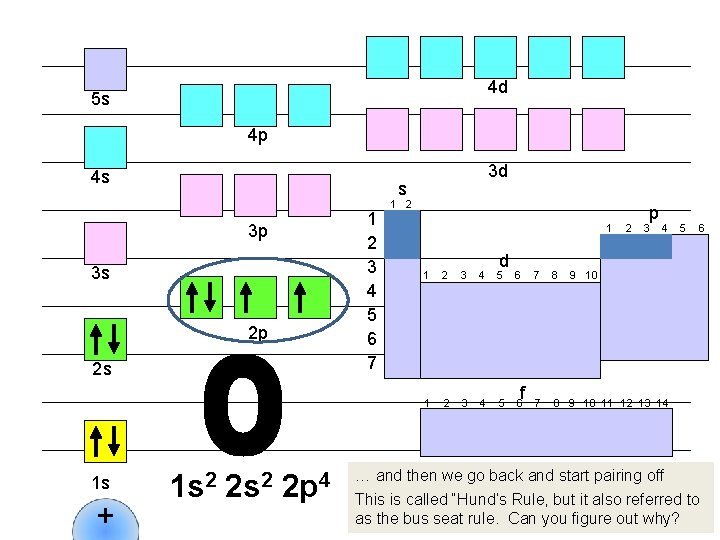
4 d 5 s 4 p 4 s s 1 2 3 4 5 6 7 3 p 3 s 2 p 2 s 1 s + 3 d 1 s 2 2 p 4 1 2 1 d 1 2 3 4 5 6 f 2 p 3 4 7 8 9 10 11 12 13 14 5 6 9 10 … and then we go back and start pairing off This is called “Hund’s Rule, but it also referred to as the bus seat rule. Can you figure out why?

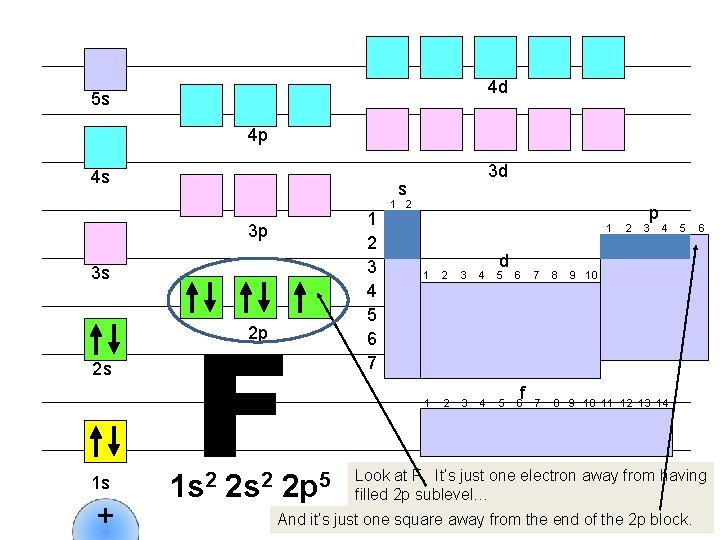
4 d 5 s 4 p 4 s s 1 2 3 4 5 6 7 3 p 3 s 2 p 2 s 1 s + 3 d 1 s 2 2 p 5 1 2 1 d 1 2 3 4 5 6 f 2 p 3 4 7 8 9 10 11 12 13 14 5 6 9 10 Look at F. It’s just one electron away from having filled 2 p sublevel… And it’s just one square away from the end of the 2 p block.

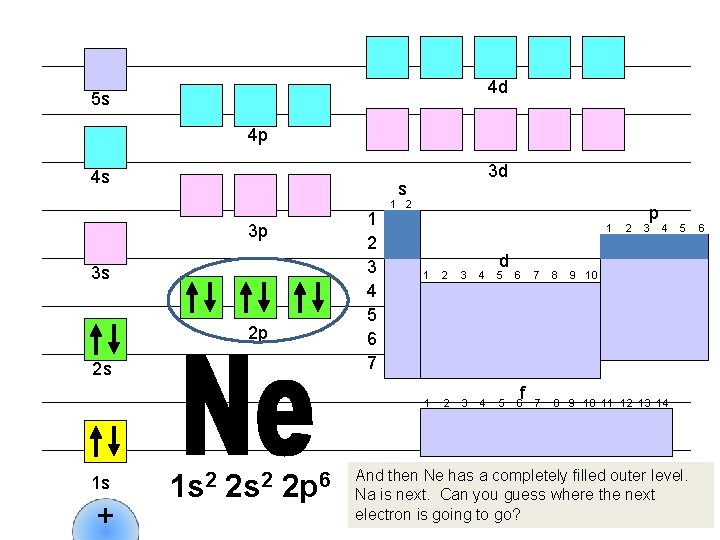
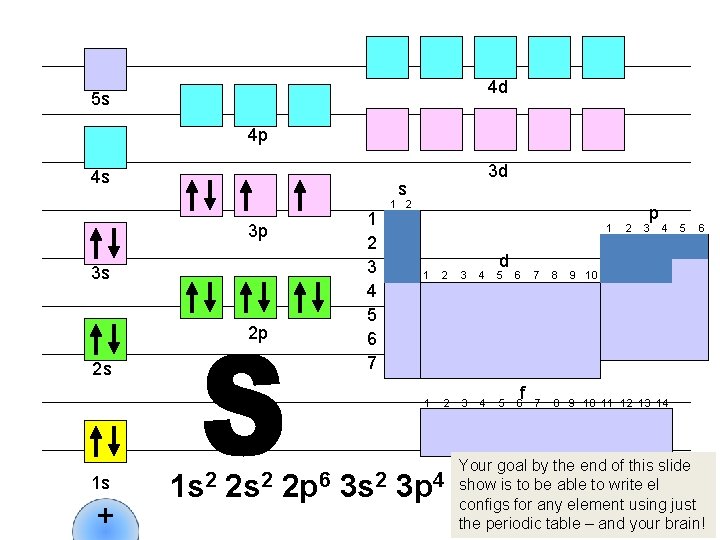
4 d 5 s 4 p 4 s s 1 2 3 4 5 6 7 3 p 3 s 2 p 2 s 1 s + 3 d 1 s 2 2 p 6 1 2 1 d 1 2 3 4 5 6 f 2 p 3 4 7 8 9 10 11 12 13 14 5 9 10 And then Ne has a completely filled outer level. Na is next. Can you guess where the next electron is going to go? 6

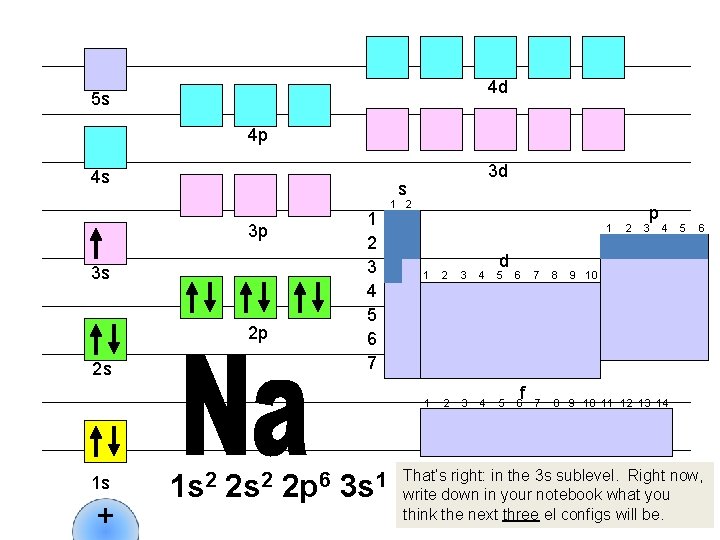
4 d 5 s 4 p 4 s s 1 2 3 4 5 6 7 3 p 3 s 2 p 2 s 1 s + 3 d 1 s 2 2 p 6 3 s 1 1 2 1 d 1 2 3 4 5 6 f 2 p 3 4 7 8 9 10 11 12 13 14 5 6 9 10 That’s right: in the 3 s sublevel. Right now, write down in your notebook what you think the next three el configs will be.

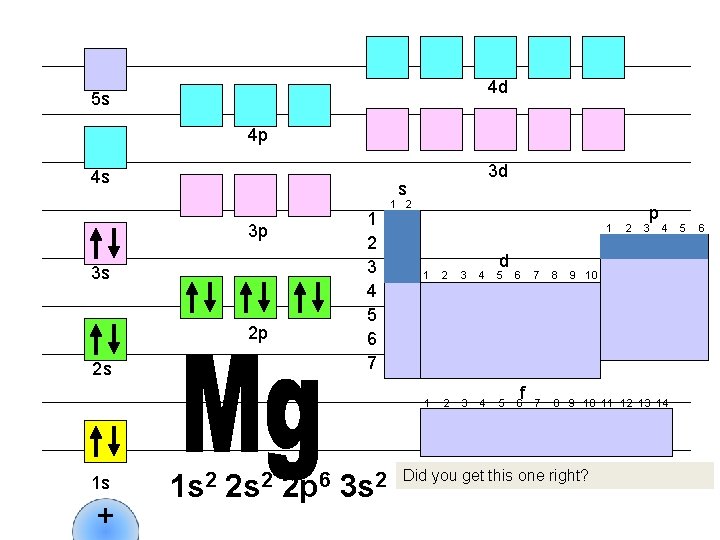
4 d 5 s 4 p 4 s s 1 2 3 4 5 6 7 3 p 3 s 2 p 2 s 1 s + 3 d 1 s 2 2 p 6 3 s 2 1 d 1 2 3 4 5 6 f 2 p 3 4 7 8 9 10 11 12 13 14 Did you get this one right? 5 6

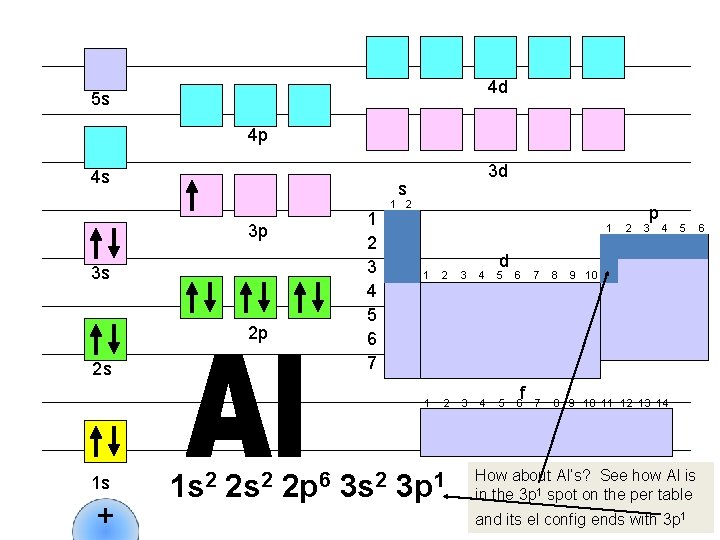
4 d 5 s 4 p 4 s s 1 2 3 4 5 6 7 3 p 3 s 2 p 2 s 1 s + 3 d 1 s 2 2 p 6 3 s 2 1 d 1 2 3 4 5 6 3 p 1 f 2 p 3 4 7 8 9 10 11 12 13 14 5 9 10 How about Al’s? See how Al is in the 3 p 1 spot on the per table and its el config ends with 3 p 1 6

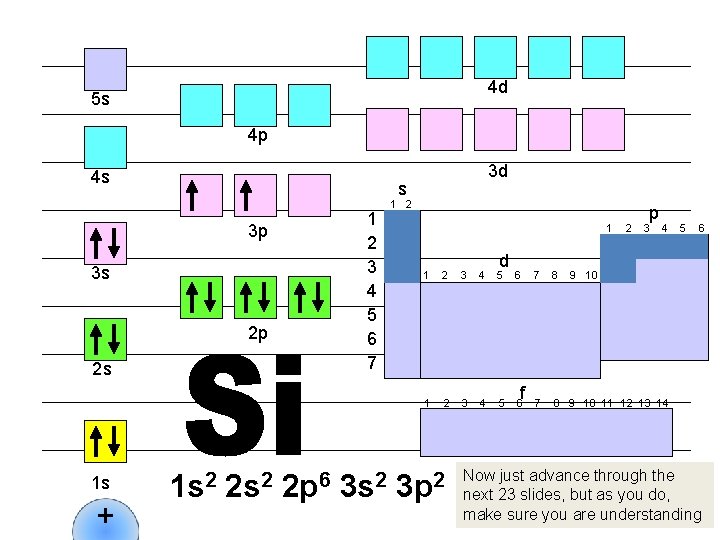
4 d 5 s 4 p 4 s s 1 2 3 4 5 6 7 3 p 3 s 2 p 2 s 1 s + 3 d 1 s 2 2 p 6 3 s 2 1 d 1 2 3 4 5 6 3 p 2 f 2 p 3 4 7 8 9 10 11 12 13 14 5 6 9 10 Now just advance through the next 23 slides, but as you do, make sure you are understanding

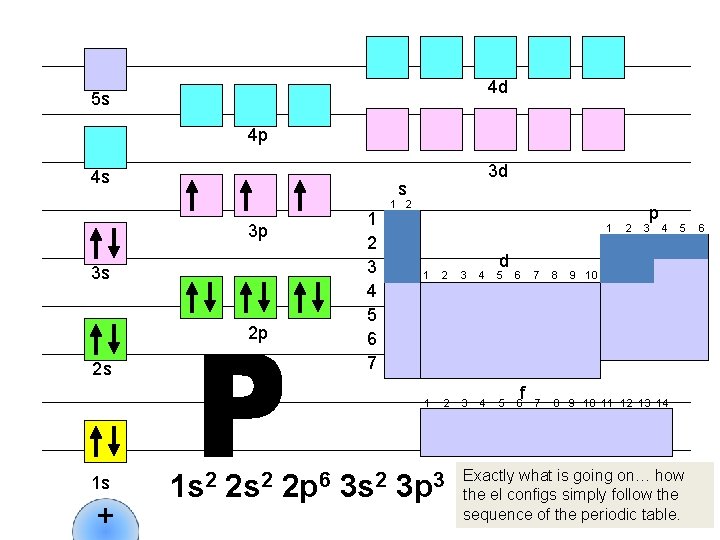
4 d 5 s 4 p 4 s s 1 2 3 4 5 6 7 3 p 3 s 2 p 2 s 1 s + 3 d 1 s 2 2 p 6 3 s 2 1 d 1 2 3 4 5 6 3 p 3 f 2 p 3 4 7 8 9 10 11 12 13 14 5 9 10 Exactly what is going on… how the el configs simply follow the sequence of the periodic table. 6

4 d 5 s 4 p 4 s s 3 p 3 s 2 p 2 s 1 s + 3 d 1 2 3 4 5 6 7 1 2 1 d 1 2 3 4 5 6 1 s 2 2 p 6 3 s 2 3 p 4 f 2 p 3 4 7 8 9 10 11 12 13 14 5 6 9 10 Your goal by the end of this slide show is to be able to write el configs for any element using just the periodic table – and your brain!

4 d 5 s 4 p 4 s s 3 p 3 s 2 p 2 s 1 s + 3 d 1 2 3 4 5 6 7 1 2 1 d 1 2 3 4 5 6 1 s 2 2 p 6 3 s 2 3 p 5 f 2 p 3 4 7 8 9 10 11 12 13 14 5 6

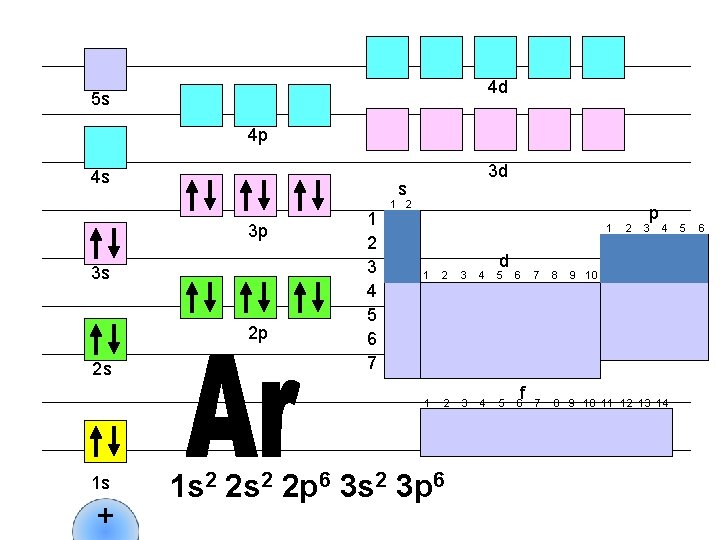
4 d 5 s 4 p 4 s s 3 p 3 s 2 p 2 s 1 s + 3 d 1 2 3 4 5 6 7 1 2 1 d 1 2 3 4 5 6 1 s 2 2 p 6 3 s 2 3 p 6 f 2 p 3 4 7 8 9 10 11 12 13 14 5 6

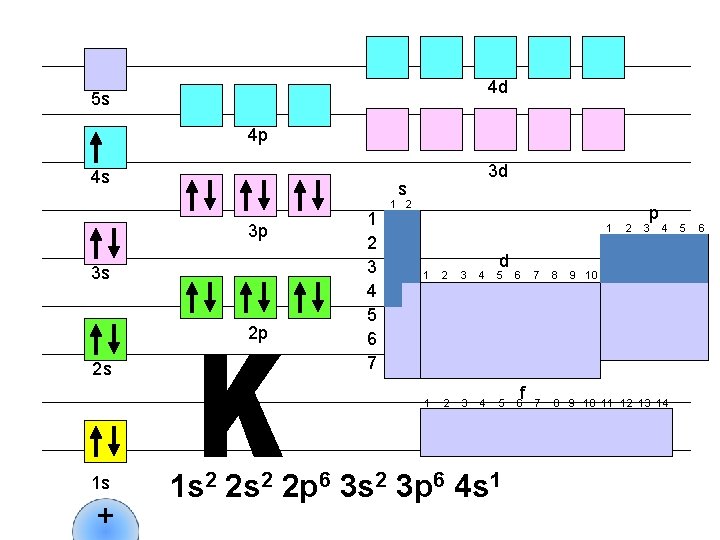
4 d 5 s 4 p 4 s s 3 p 3 s 2 p 2 s 1 s + 3 d 1 2 3 4 5 6 7 1 2 1 d 1 2 3 4 5 6 1 s 2 2 p 6 3 s 2 3 p 6 4 s 1 f 2 p 3 4 7 8 9 10 11 12 13 14 5 6

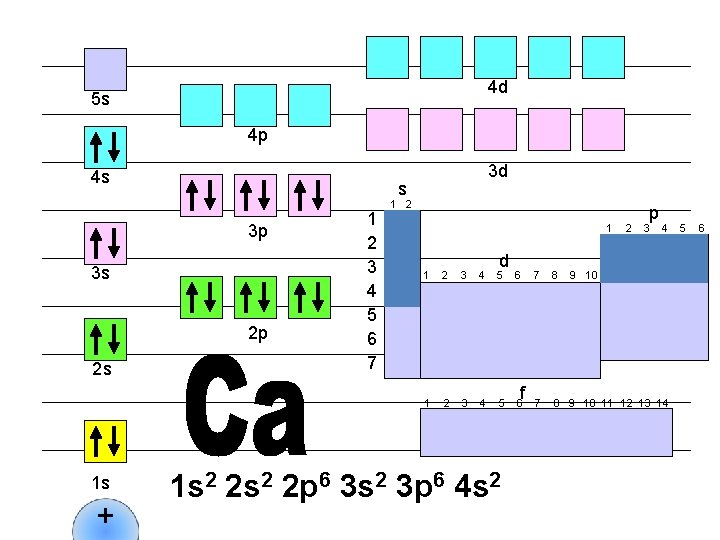
4 d 5 s 4 p 4 s s 3 p 3 s 2 p 2 s 1 s + 3 d 1 2 3 4 5 6 7 1 2 1 d 1 2 3 4 5 6 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 f 2 p 3 4 7 8 9 10 11 12 13 14 5 6

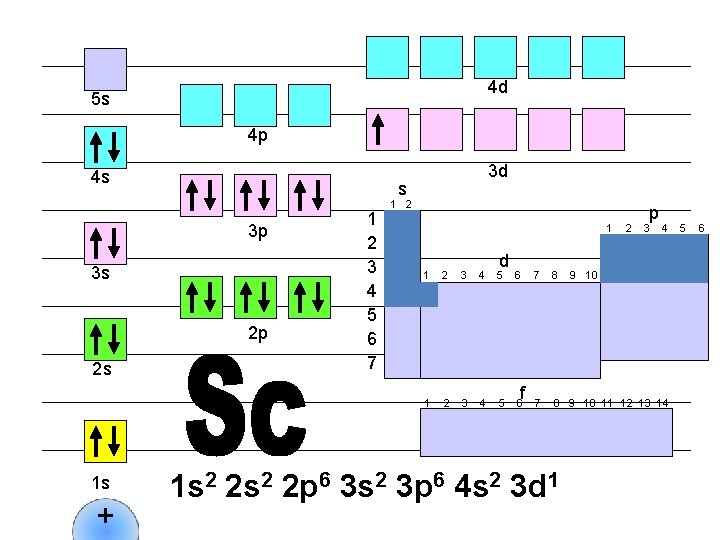
4 d 5 s 4 p 4 s s 3 p 3 s 2 p 2 s 1 s + 3 d 1 2 3 4 5 6 7 1 2 1 d 1 2 3 4 5 6 f 2 p 3 4 7 8 9 10 11 12 13 14 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 1 9 10 5 6

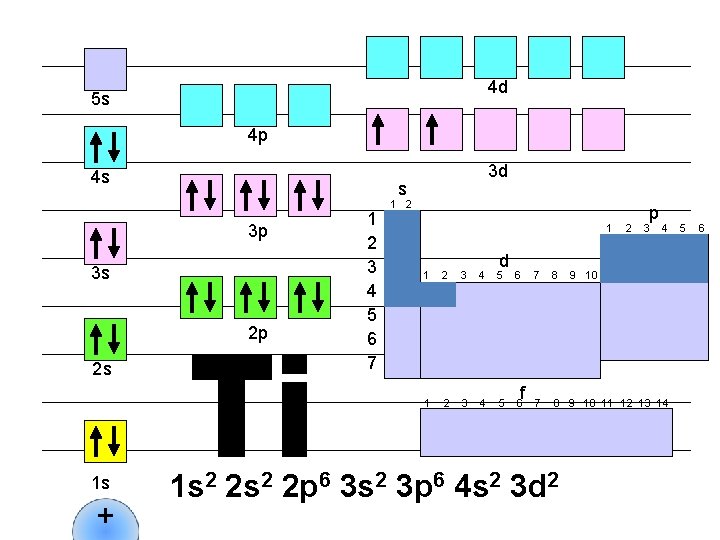
4 d 5 s 4 p 4 s s 3 p 3 s 2 p 2 s 1 s + 3 d 1 2 3 4 5 6 7 1 2 1 d 1 2 3 4 5 6 f 2 p 3 4 7 8 9 10 11 12 13 14 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 2 9 10 5 6

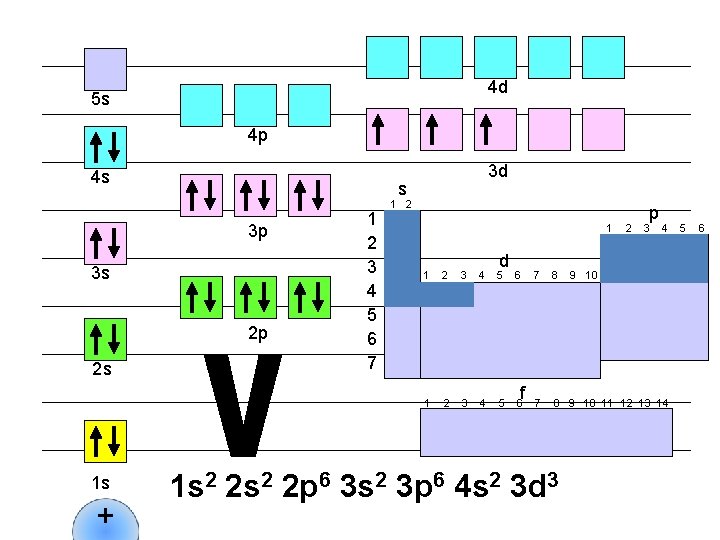
4 d 5 s 4 p 4 s s 3 p 3 s 2 p 2 s 1 s + 3 d 1 2 3 4 5 6 7 1 2 1 d 1 2 3 4 5 6 f 2 p 3 4 7 8 9 10 11 12 13 14 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 3 9 10 5 6

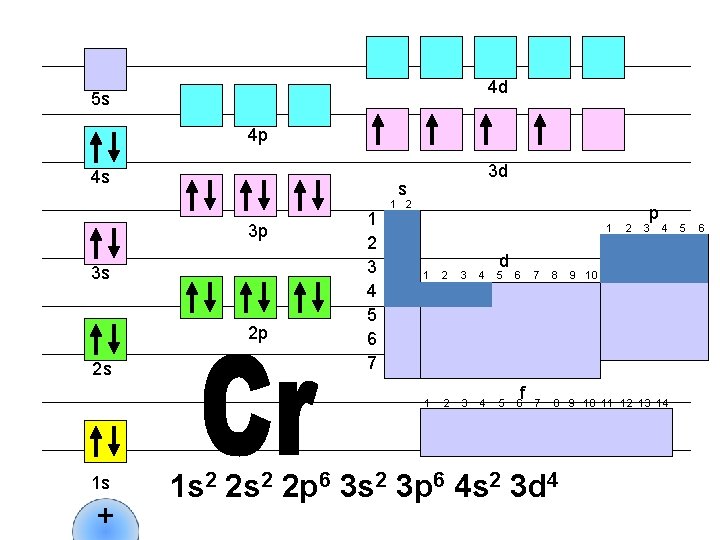
4 d 5 s 4 p 4 s s 3 p 3 s 2 p 2 s 1 s + 3 d 1 2 3 4 5 6 7 1 2 1 d 1 2 3 4 5 6 f 2 p 3 4 7 8 9 10 11 12 13 14 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 4 9 10 5 6

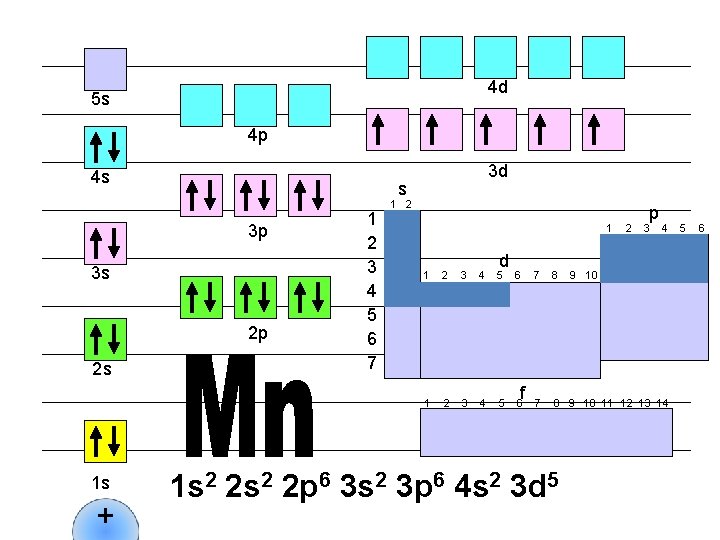
4 d 5 s 4 p 4 s s 3 p 3 s 2 p 2 s 1 s + 3 d 1 2 3 4 5 6 7 1 2 1 d 1 2 3 4 5 6 f 2 p 3 4 7 8 9 10 11 12 13 14 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 5 9 10 5 6

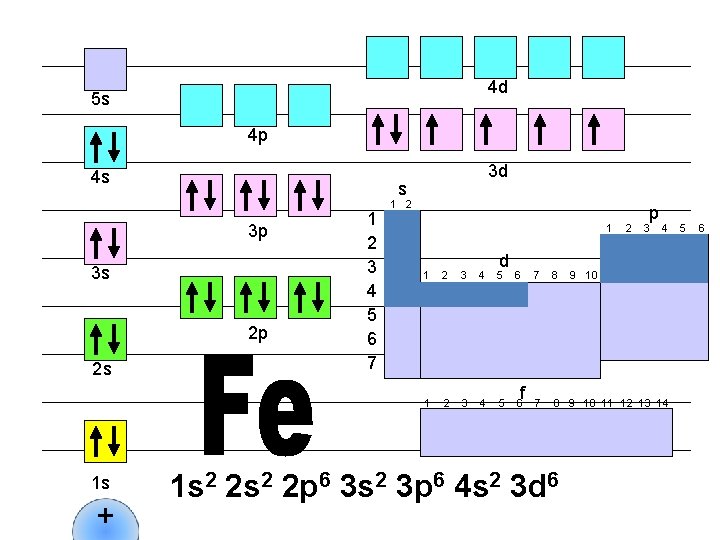
4 d 5 s 4 p 4 s s 3 p 3 s 2 p 2 s 1 s + 3 d 1 2 3 4 5 6 7 1 2 1 d 1 2 3 4 5 6 f 2 p 3 4 7 8 9 10 11 12 13 14 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 6 9 10 5 6

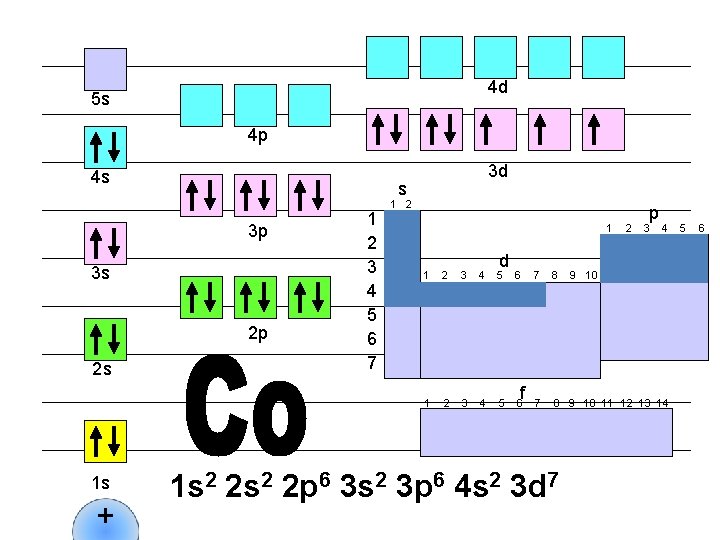
4 d 5 s 4 p 4 s s 3 p 3 s 2 p 2 s 1 s + 3 d 1 2 3 4 5 6 7 1 2 1 d 1 2 3 4 5 6 f 2 p 3 4 7 8 9 10 11 12 13 14 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 7 9 10 5 6

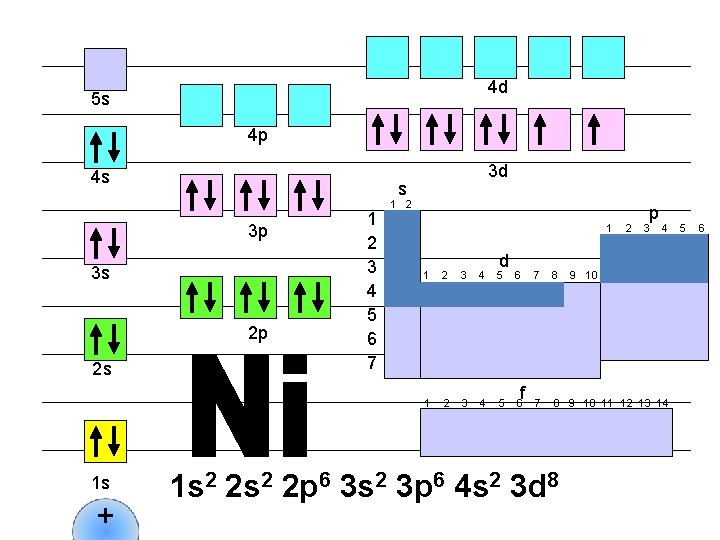
4 d 5 s 4 p 4 s s 3 p 3 s 2 p 2 s 1 s + 3 d 1 2 3 4 5 6 7 1 2 1 d 1 2 3 4 5 6 f 2 p 3 4 7 8 9 10 11 12 13 14 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 8 9 10 5 6

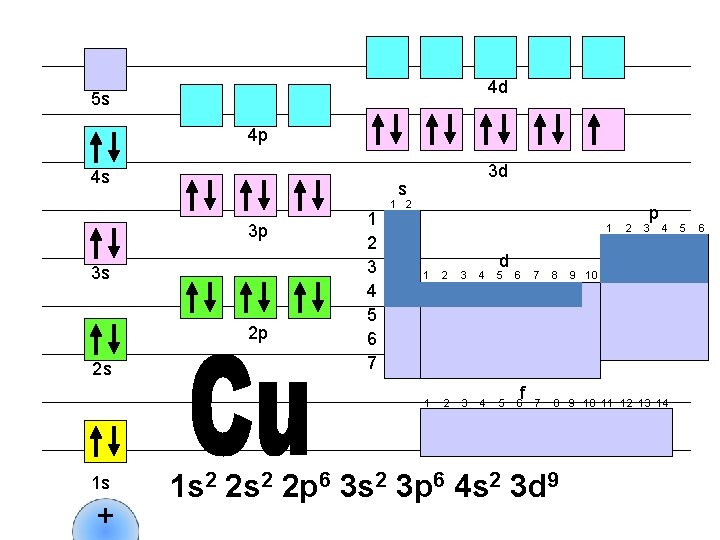
4 d 5 s 4 p 4 s s 3 p 3 s 2 p 2 s 1 s + 3 d 1 2 3 4 5 6 7 1 2 1 d 1 2 3 4 5 6 f 2 p 3 4 7 8 9 10 11 12 13 14 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 9 9 10 5 6

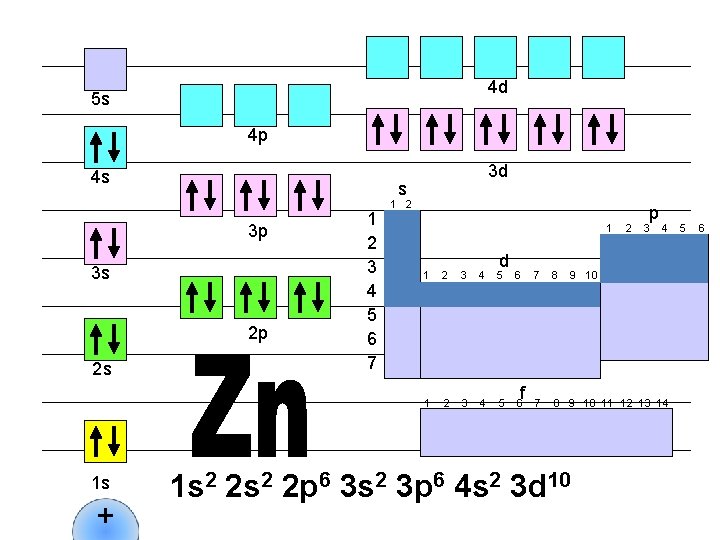
4 d 5 s 4 p 4 s s 3 p 3 s 2 p 2 s 1 s + 3 d 1 2 3 4 5 6 7 1 2 1 d 1 2 3 4 5 6 f 2 p 3 4 7 8 9 10 11 12 13 14 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 5 6

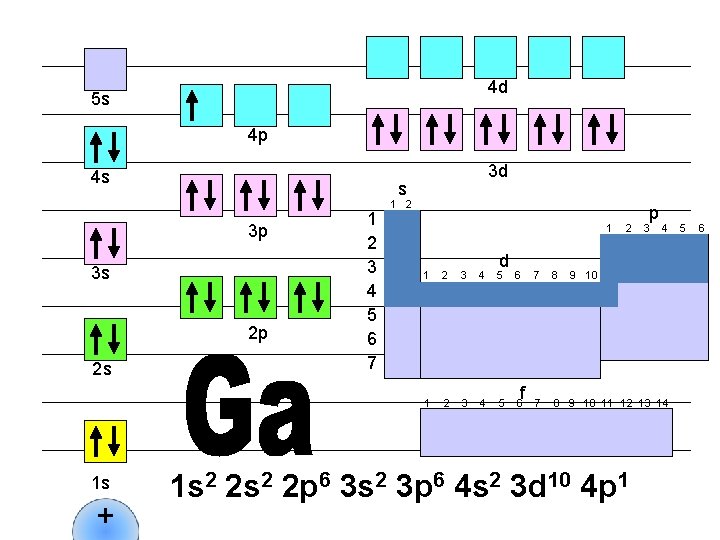
4 d 5 s 4 p 4 s s 3 p 3 s 2 p 2 s 1 s + 3 d 1 2 3 4 5 6 7 1 2 1 d 1 2 3 4 5 6 f 2 p 3 4 7 8 9 10 11 12 13 14 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 1 5 6

4 d 5 s 4 p 4 s s 3 p 3 s 2 p 2 s 1 s + 3 d 1 2 3 4 5 6 7 1 2 1 d 1 2 3 4 5 6 f 2 p 3 4 7 8 9 10 11 12 13 14 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 2 5 6

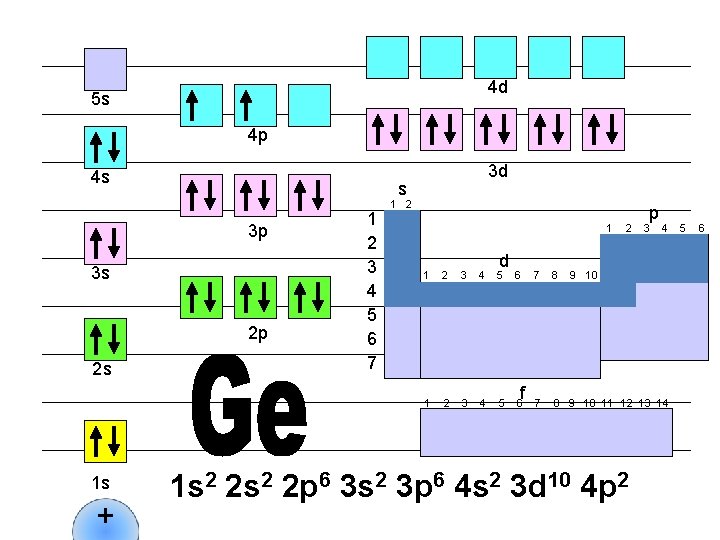
4 d 5 s 4 p 4 s s 3 p 3 s 2 p 2 s 1 s + 3 d 1 2 3 4 5 6 7 1 2 1 d 1 2 3 4 5 6 f 2 p 3 4 7 8 9 10 11 12 13 14 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 3 5 6

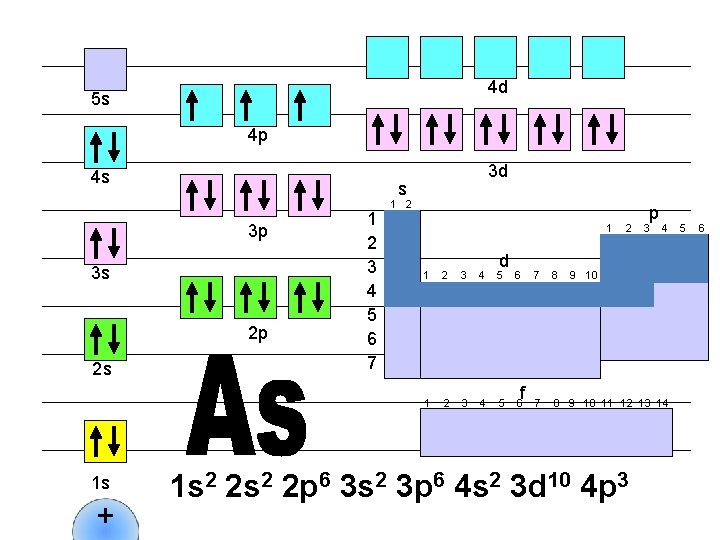
4 d 5 s 4 p 4 s s 3 p 3 s 2 p 2 s 1 s + 3 d 1 2 3 4 5 6 7 1 2 1 d 1 2 3 4 5 6 f 2 p 3 4 7 8 9 10 11 12 13 14 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 4 5 6

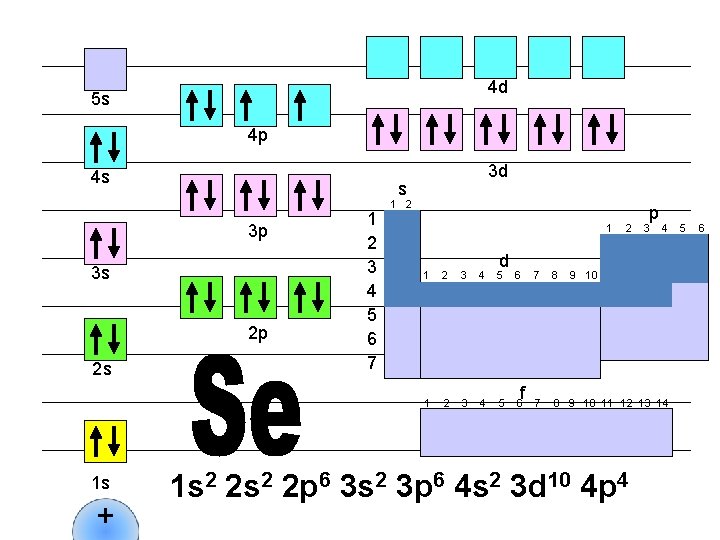
4 d 5 s 4 p 4 s s 3 p 3 s 2 p 2 s 1 s + 3 d 1 2 3 4 5 6 7 1 2 1 d 1 2 3 4 5 6 f 2 p 3 4 7 8 9 10 11 12 13 14 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 5 5 6

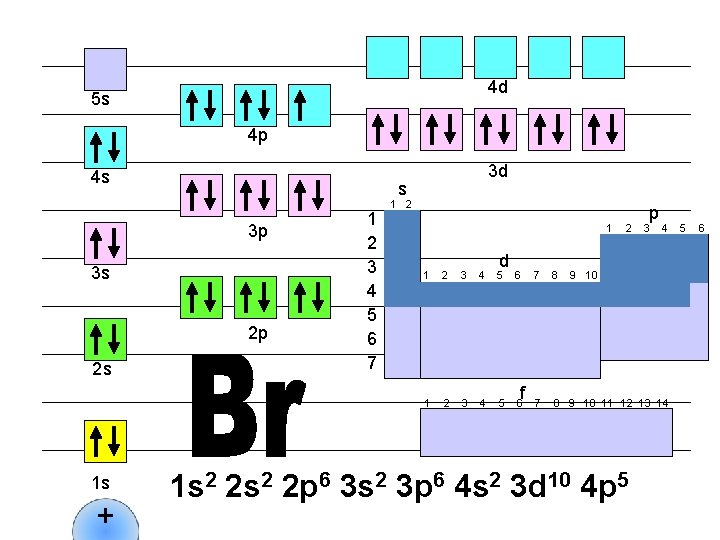
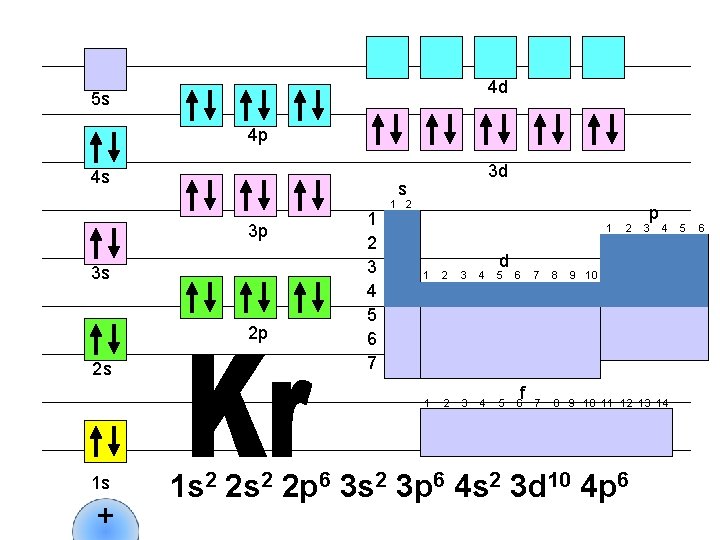
4 d 5 s 4 p 4 s s 3 p 3 s 2 p 2 s 1 s + 3 d 1 2 3 4 5 6 7 1 2 1 d 1 2 3 4 5 6 f 2 p 3 4 7 8 9 10 11 12 13 14 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 6

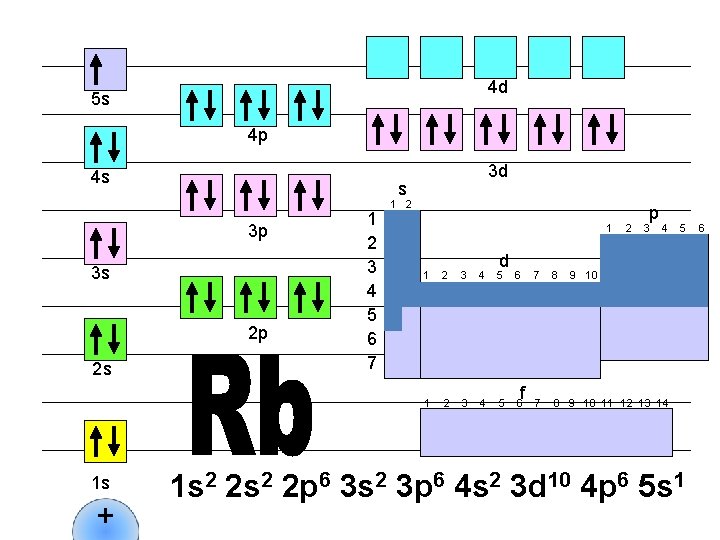
4 d 5 s 4 p 4 s s 3 p 3 s 2 p 2 s 1 s + 3 d 1 2 3 4 5 6 7 1 2 1 d 1 2 3 4 5 6 f 2 p 3 4 7 8 9 10 11 12 13 14 5 9 10 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 s 1 6

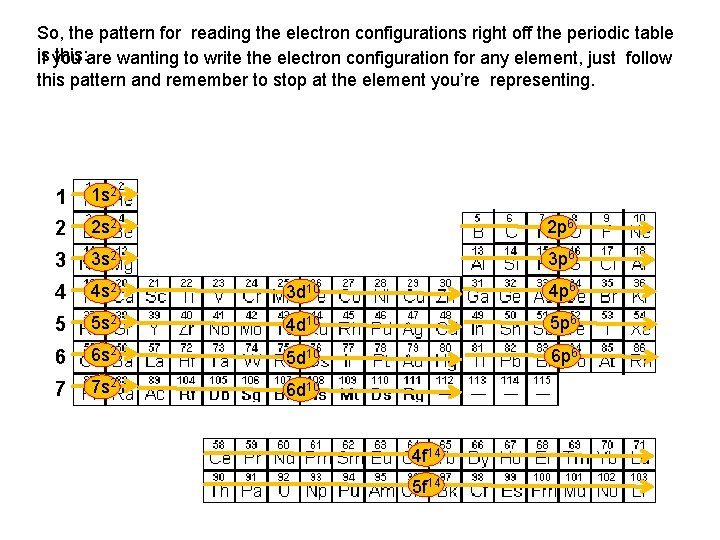
So, the pattern for reading the electron configurations right off the periodic table is you this: are wanting to write the electron configuration for any element, just follow If this pattern and remember to stop at the element you’re representing. 1 1 s 2 2 2 s 2 2 p 6 3 3 s 2 3 p 6 4 4 s 2 3 d 10 4 p 6 5 5 s 2 4 d 10 5 p 6 6 6 s 2 5 d 10 6 p 6 7 7 s 2 6 d 10 4 f 14 5 f 14

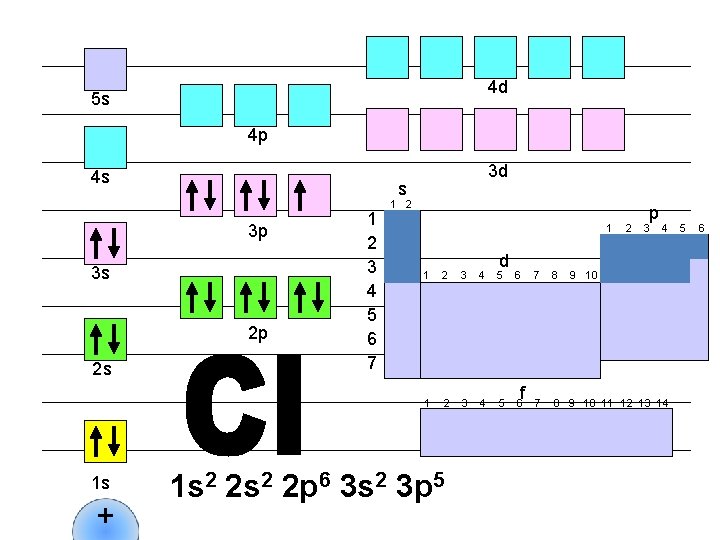
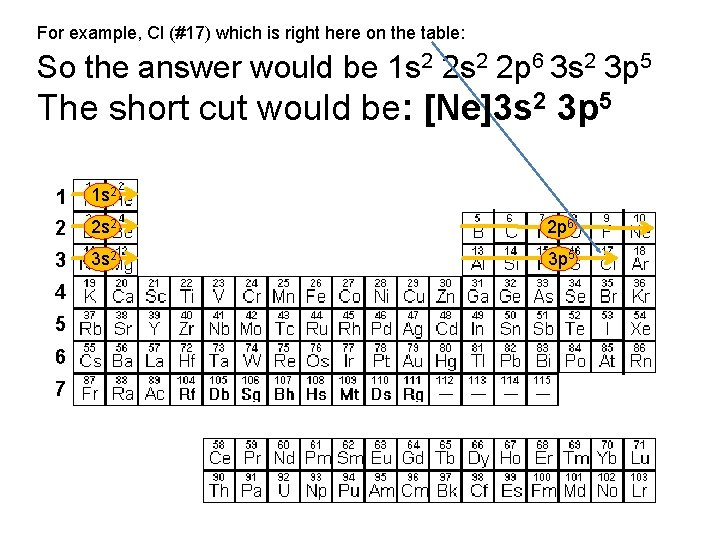
For example, Cl (#17) which is right here on the table: So the answer would be 1 s 2 2 p 6 3 s 2 3 p 5 The short cut would be: [Ne]3 s 2 3 p 5 1 1 s 2 2 2 s 2 2 p 6 3 3 s 2 3 p 5 4 5 6 7

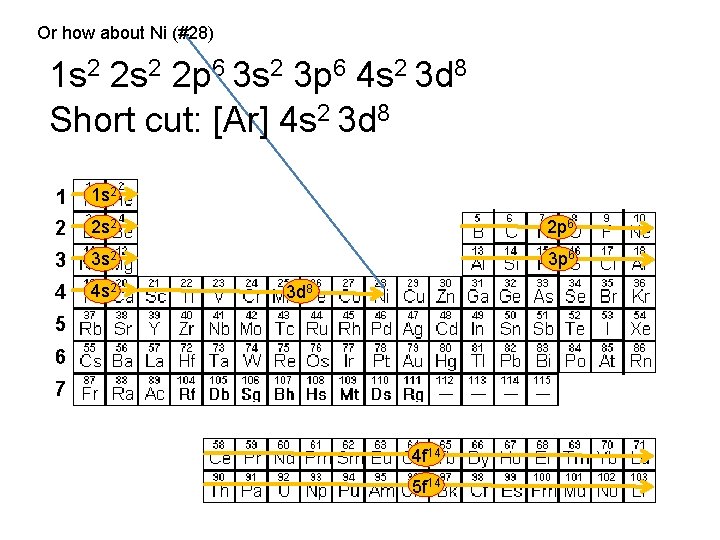
Or how about Ni (#28) 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 8 Short cut: [Ar] 4 s 2 3 d 8 1 1 s 2 2 2 s 2 2 p 6 3 3 s 2 3 p 6 4 4 s 2 3 d 8 5 6 7 4 f 14 5 f 14

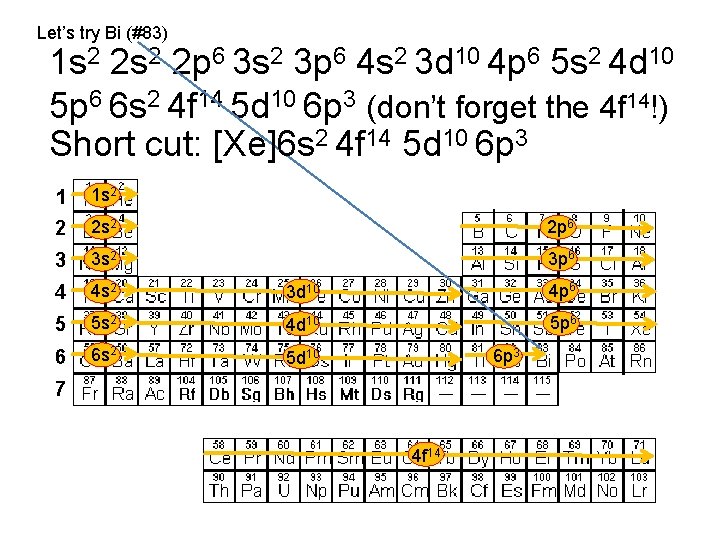
Let’s try Bi (#83) 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 s 2 4 d 10 5 p 6 6 s 2 4 f 14 5 d 10 6 p 3 (don’t forget the 4 f 14!) Short cut: [Xe]6 s 2 4 f 14 5 d 10 6 p 3 1 1 s 2 2 2 s 2 2 p 6 3 3 s 2 3 p 6 4 4 s 2 3 d 10 4 p 6 5 5 s 2 4 d 10 5 p 6 6 6 s 2 5 d 10 6 p 3 7 4 f 14

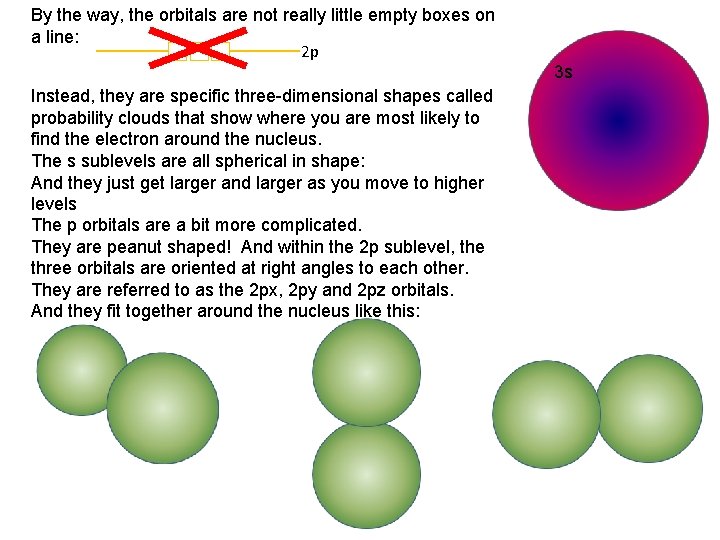
By the way, the orbitals are not really little empty boxes on a line: 2 p Instead, they are specific three-dimensional shapes called probability clouds that show where you are most likely to find the electron around the nucleus. The s sublevels are all spherical in shape: And they just get larger and larger as you move to higher levels The p orbitals are a bit more complicated. They are peanut shaped! And within the 2 p sublevel, the three orbitals are oriented at right angles to each other. They are referred to as the 2 px, 2 py and 2 pz orbitals. And they fit together around the nucleus like this: 3 s 2 s 1 s

Now try some of the problems from the Electron Configuartion worksheet. The End