Electron Configurations Valence A Lewis electron dot structures



- Slides: 19

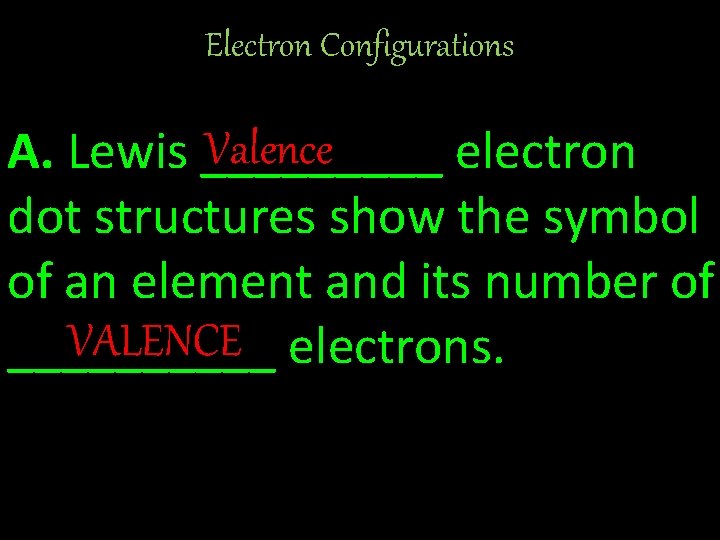
Electron Configurations Valence A. Lewis _____ electron dot structures show the symbol of an element and its number of VALENCE electrons. _____

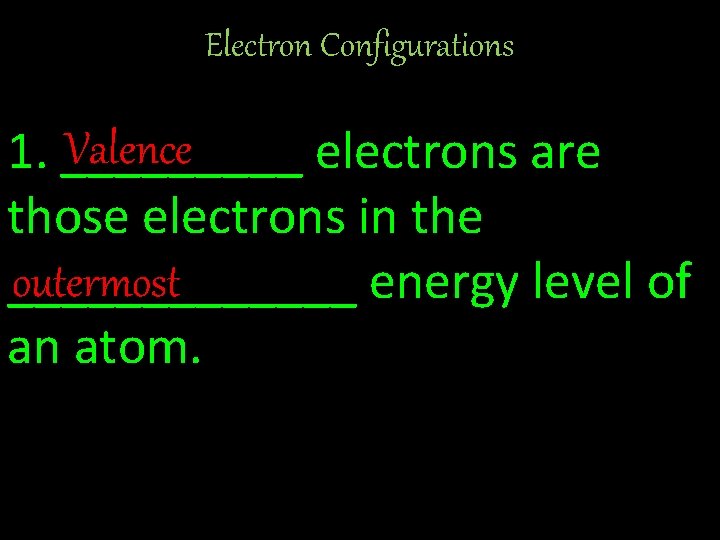
Electron Configurations Valence 1. _____ electrons are those electrons in the outermost _______ energy level of an atom.

Electron Configurations Valence 2. _____ electrons are integral in determining how the chemically atom will _______ react with other atoms.

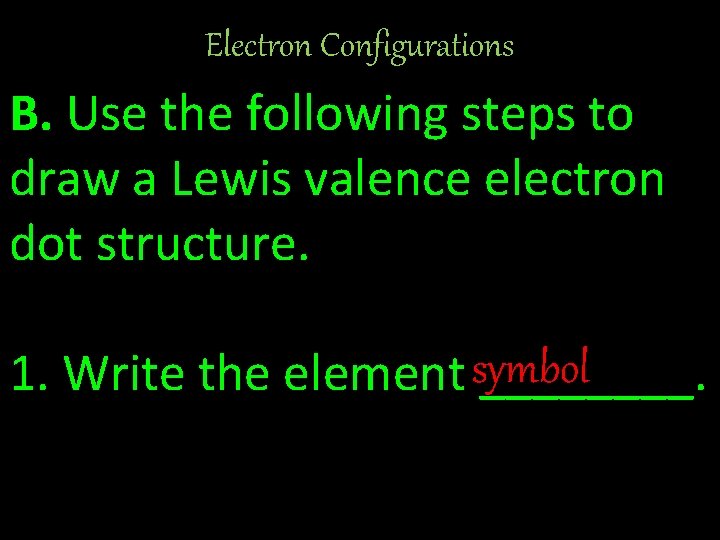
Electron Configurations B. Use the following steps to draw a Lewis valence electron dot structure. 1. Write the element symbol ____.

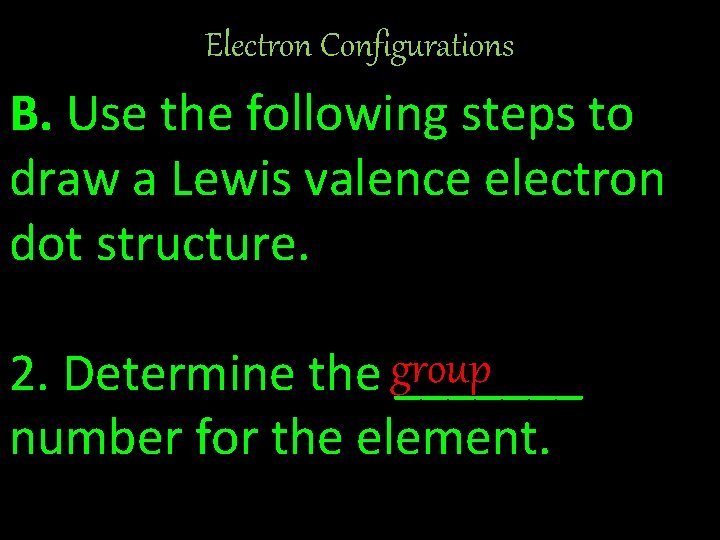
Electron Configurations B. Use the following steps to draw a Lewis valence electron dot structure. 2. Determine the group _______ number for the element.

Electron Configurations B. Use the following steps to draw a Lewis valence electron dot structure. a. The group _______ number indicates the number of VALENCE _____ electrons.

Electron Configurations B. Use the following steps to draw a Lewis valence electron dot structure. right 3. Start on the _____ of your element symbol and, moving counterdot every 90° until clockwise, put a ______ the number of valence electrons present in the atom is achieved.


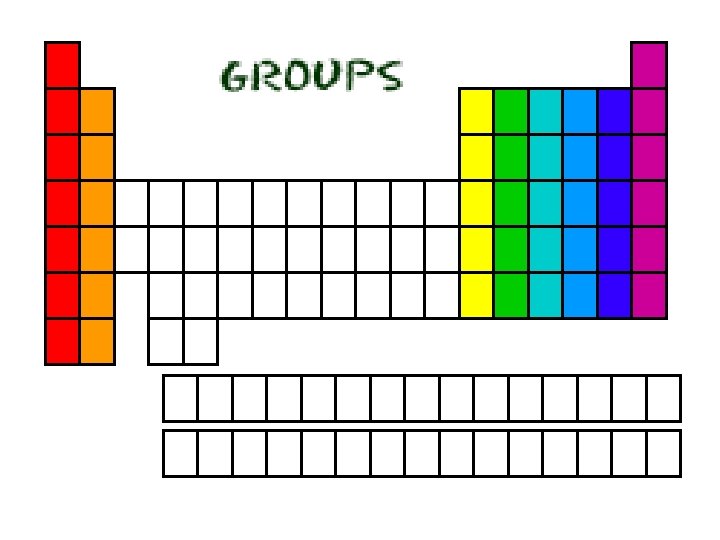
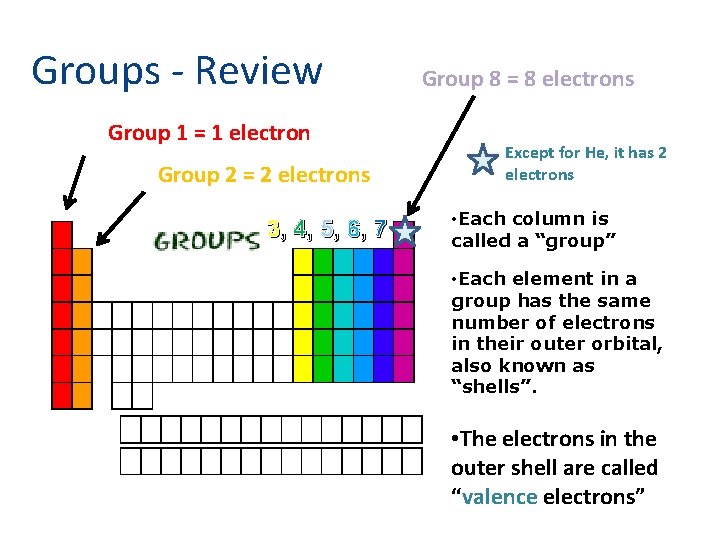
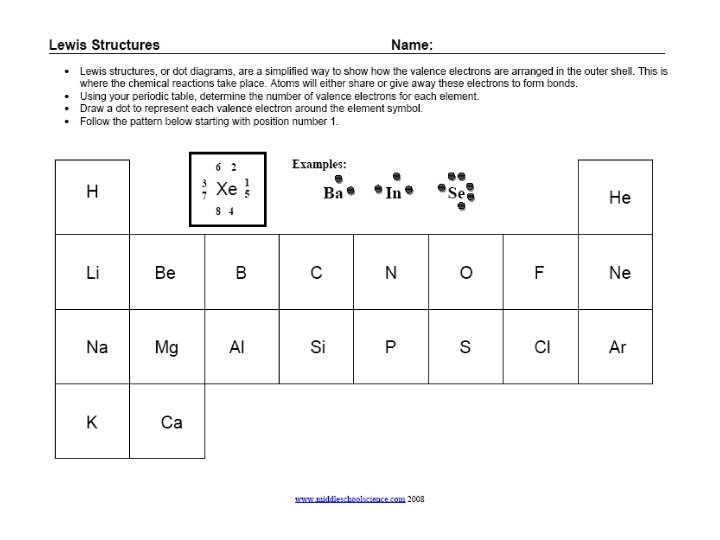
Groups - Review Group 1 = 1 electron Group 2 = 2 electrons 3, 4, 5, 6, 7 Group 8 = 8 electrons Except for He, it has 2 electrons • Each column is called a “group” • Each element in a group has the same number of electrons in their outer orbital, also known as “shells”. • The electrons in the outer shell are called “valence electrons”

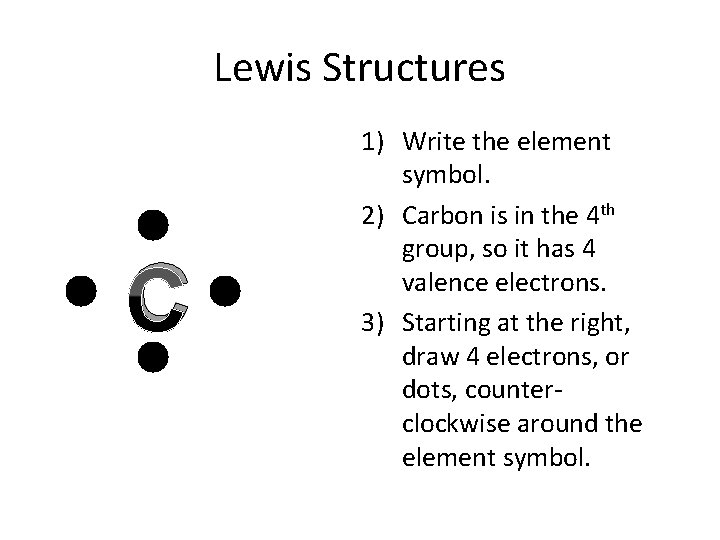
Lewis Structures C 1) Write the element symbol. 2) Carbon is in the 4 th group, so it has 4 valence electrons. 3) Starting at the right, draw 4 electrons, or dots, counterclockwise around the element symbol.

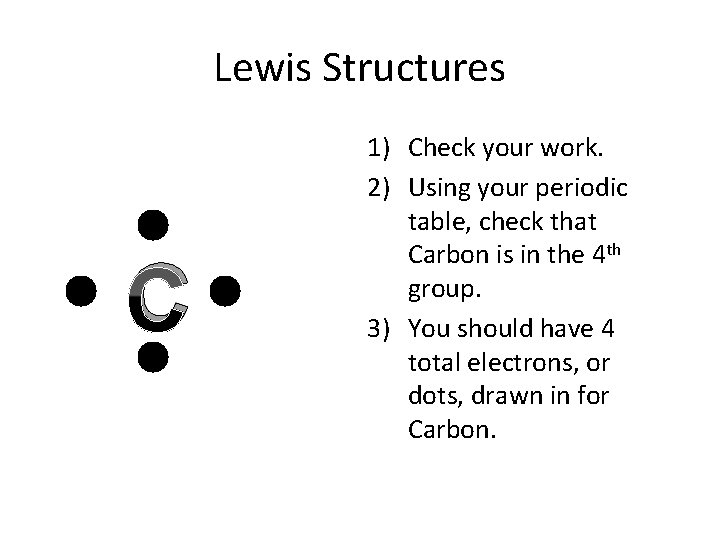
Lewis Structures C 1) Check your work. 2) Using your periodic table, check that Carbon is in the 4 th group. 3) You should have 4 total electrons, or dots, drawn in for Carbon.

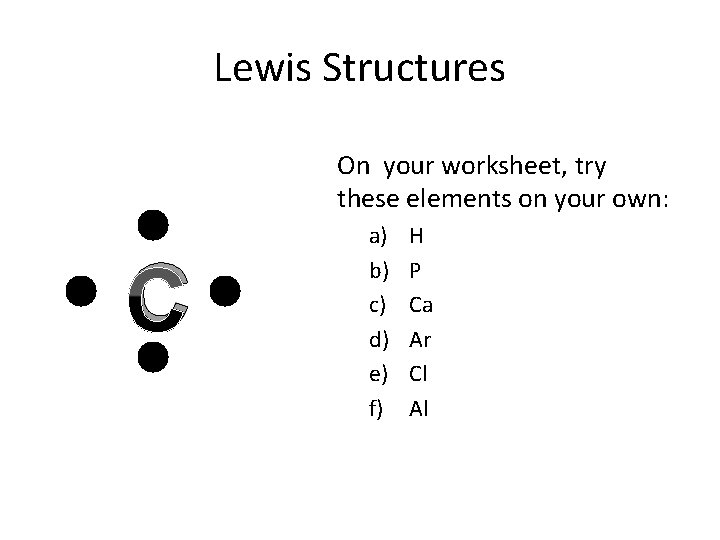
Lewis Structures On your worksheet, try these elements on your own: C a) b) c) d) e) f) H P Ca Ar Cl Al

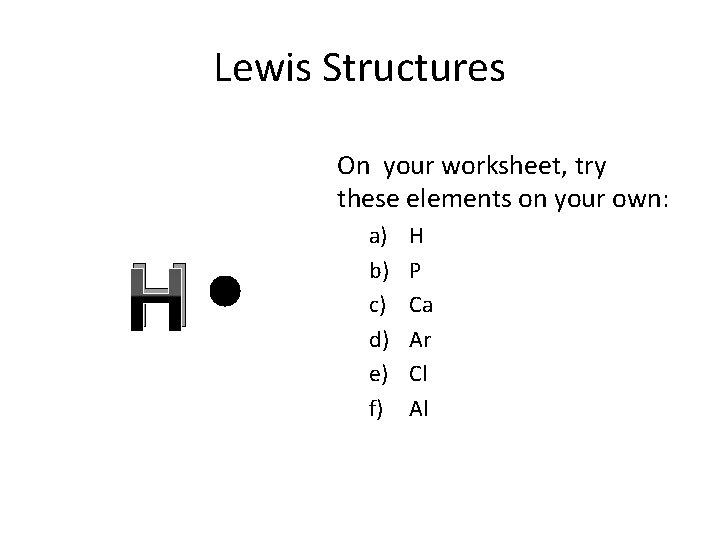
Lewis Structures On your worksheet, try these elements on your own: H a) b) c) d) e) f) H P Ca Ar Cl Al

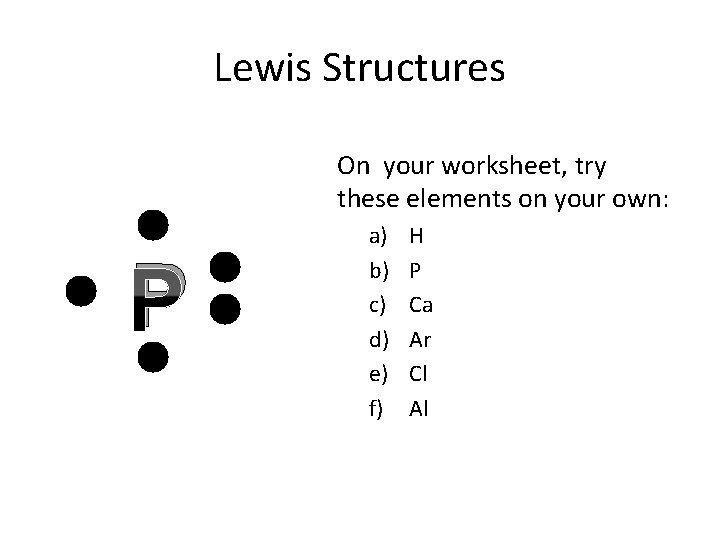
Lewis Structures On your worksheet, try these elements on your own: P a) b) c) d) e) f) H P Ca Ar Cl Al

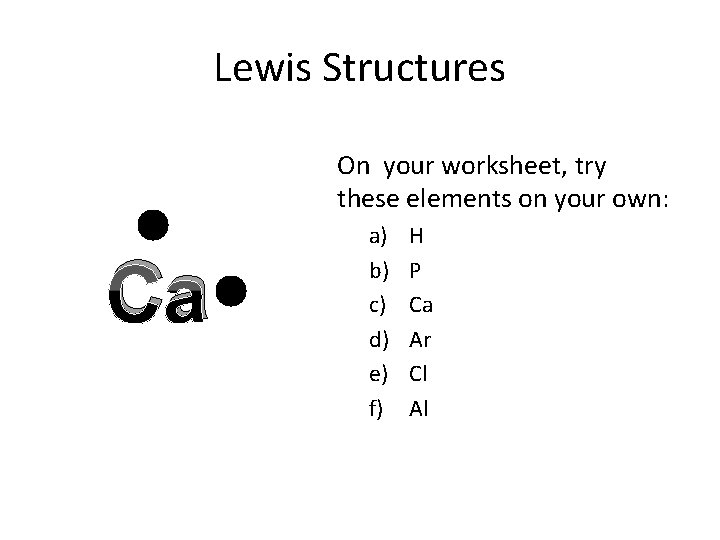
Lewis Structures On your worksheet, try these elements on your own: Ca a) b) c) d) e) f) H P Ca Ar Cl Al

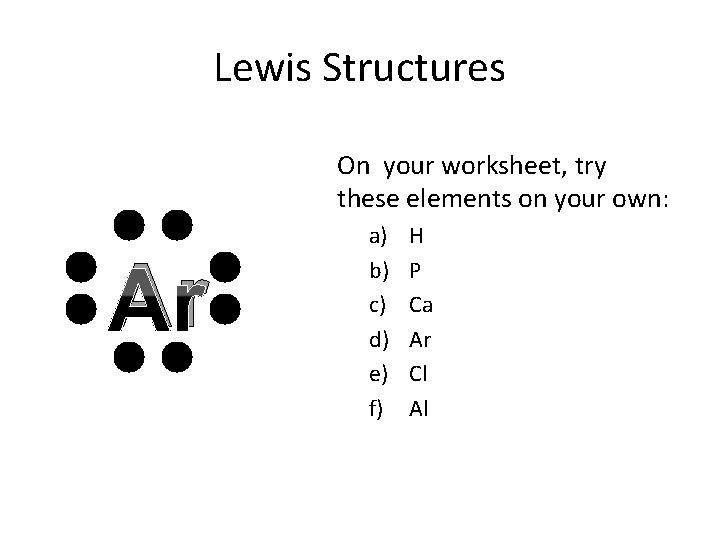
Lewis Structures On your worksheet, try these elements on your own: Ar a) b) c) d) e) f) H P Ca Ar Cl Al

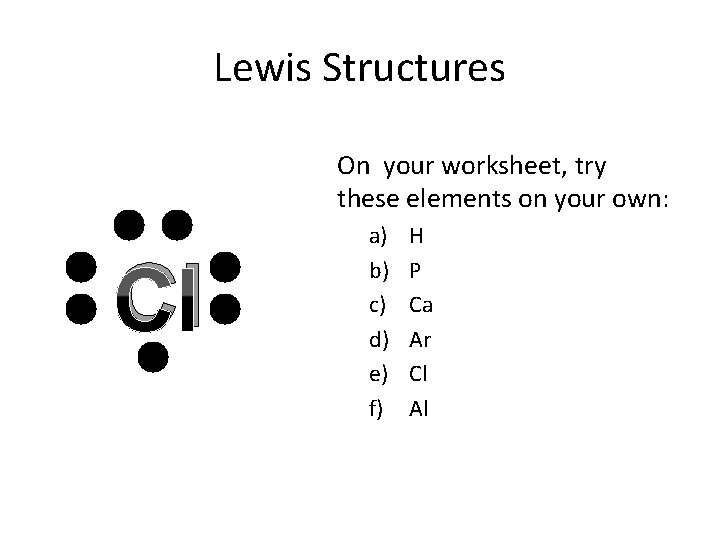
Lewis Structures On your worksheet, try these elements on your own: Cl a) b) c) d) e) f) H P Ca Ar Cl Al

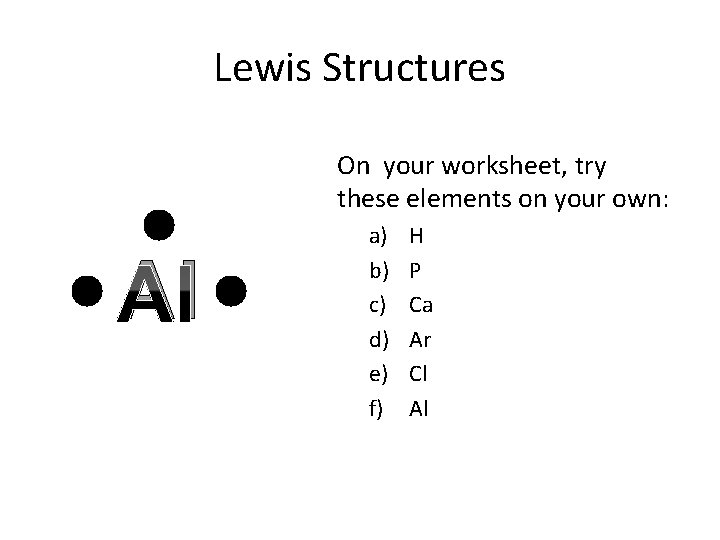
Lewis Structures On your worksheet, try these elements on your own: Al a) b) c) d) e) f) H P Ca Ar Cl Al
