Electron Configurations THE QUANTUM MECHANICAL MODEL OF THE






- Slides: 23

Electron Configurations THE QUANTUM MECHANICAL MODEL OF THE ATOM

Quantum Mechanical Model �Developed by Erwin Schrodinger �aka “Electron Cloud” model �Doesn’t define an exact path of electron; estimates probability of finding electron in a certain location �Uses atomic orbitals = a 3 -D region around nucleus that describes the electron’s probable location. Each orbital can hold a maximum of 2 electrons

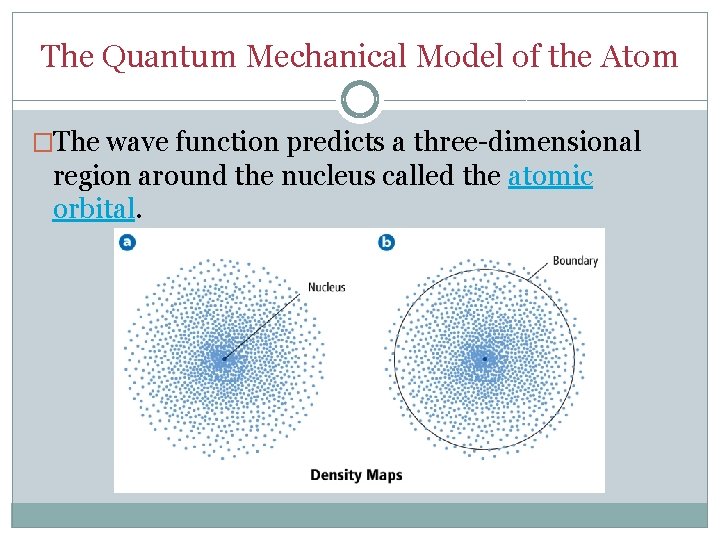
The Quantum Mechanical Model of the Atom �The wave function predicts a three-dimensional region around the nucleus called the atomic orbital.

Atomic Orbitals �Electrons cannot exist between energy levels (just like the rungs of a ladder). �Principal quantum number (n) indicates the relative size and energy of atomic orbitals. n specifies the atom’s major energy levels, called the principal energy levels.

Other Quantum Numbers (QN) �Angular Momentum QN (l = 0 to n 1) - relates to shape of the orbital. �Magnetic QN (ml = l to l) - relates to orientation of the orbital in space relative to other orbitals. �Electron Spin QN (ms = +1/2, 1/2) - relates to the spin states of the electrons.

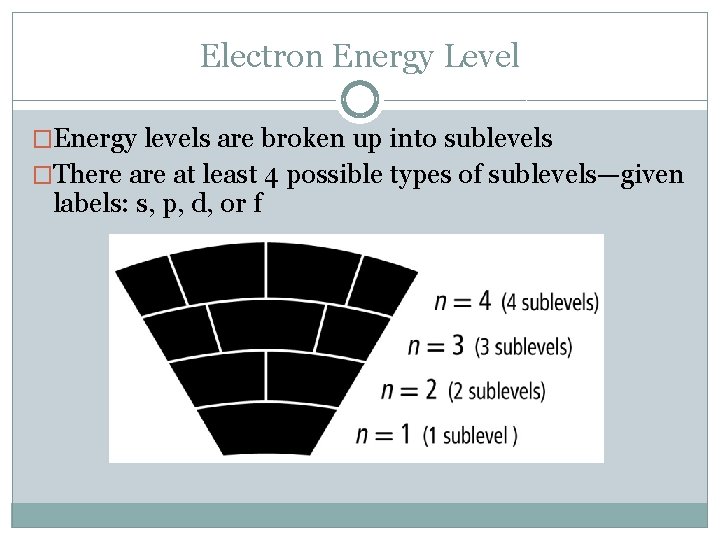
Electron Energy Level �Energy levels are broken up into sublevels �There at least 4 possible types of sublevels—given labels: s, p, d, or f

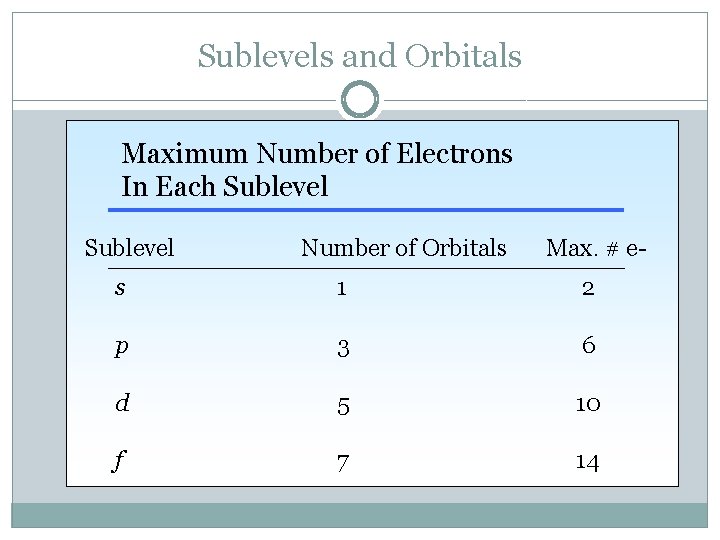
Sublevels and Orbitals Maximum Number of Electrons In Each Sublevel Number of Orbitals Max. # e- s 1 2 p 3 6 d 5 10 f 7 14

Shapes of Orbitals

Electron Configurations �The electron configuration of an atom is the arrangement of the electrons around the nucleus of an atom. �RULES: �Aufbau Principle: Electrons are added one at a time to the lowest energy orbitals available until all the electrons of the atom have been accounted for.

Rules Continued �Pauli Exclusion Principle: In a given atom, no two electrons can have the same set of four quantum numbers (n, l, ms). Therefore, an orbital can hold only two electrons, and they must have opposite spins. �Hund’s Rule: Electrons occupy equalenergy orbitals so that a maximum number of unpaired electrons results.

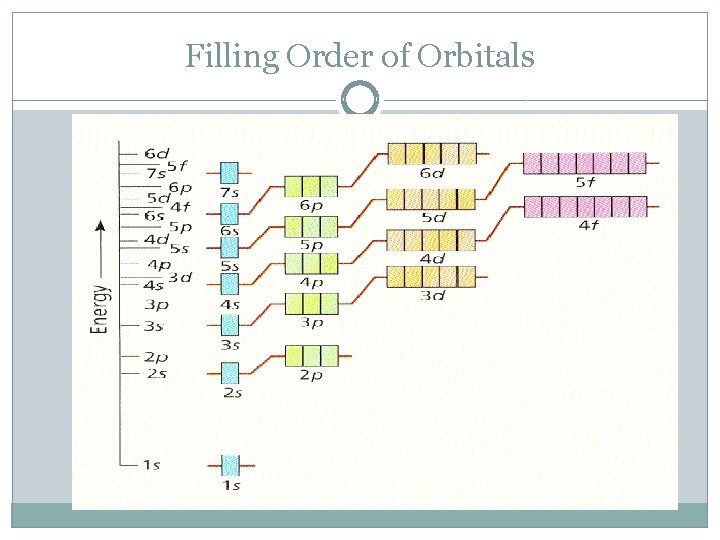
Filling Order of Orbitals

Example of Electron Configurations using Aufbau Box Diagrams 1. Hydrogen 2. Lithium 3. Carbon

More Examples �Iron: �Sulfur

“Short Version” Electron Configurations �Write the Aufbau box diagram e- configuration for phosphorus: The “short version” would be:

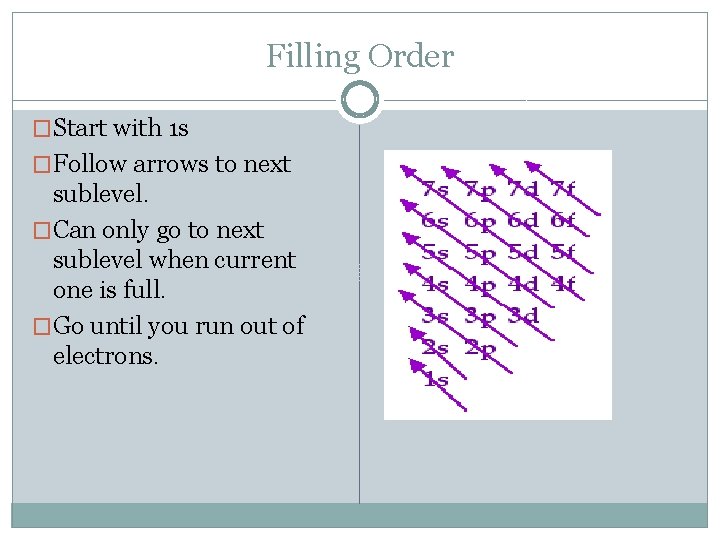
Filling Order �Start with 1 s �Follow arrows to next sublevel. �Can only go to next sublevel when current one is full. �Go until you run out of electrons.

Examples 1. Sulfur 2. Strontium 3. Antimony

Noble Gas Abbreviated e- Configurations �The symbol for a Noble Gas (Group 18 elements) can be used to shorten the configuration. Choose the noble gas ending the period (row) above the element in question. �Example:

Valence Electrons �The electrons occupying the outermost energy levels of an atom �Located in the highest occupied s and p sublevel Maximum Number = 8 �Determined by the location of the element on the periodic table. �Determine the physical and chemical properties of the element

Finding # of Valence Electrons �Group #1 = 1 valence electrons �Group #2 = 2 valence electrons �For Groups #13 -18 Subtract 10 from the group # = # valence electrons Exception Helium only has 2

Noble Gas Stability �Noble gases are usually unreactive �This is because they have max. # valence electrons �For two atoms to join together atoms must gain, lose or share valence electrons �Elements with max. # of valence electrons do not easily gain or lose electrons

Practice Problems �Determine the number of valence electrons for the following elements: Sodium Chlorine Neon Magnesium Aluminum

Electron (Lewis) Dot Diagrams �Model used to display the valence electrons of an element. �Includes the symbol of the element and the valence electrons represented as dots. �Example: Phosphorus

Practice Problems �Draw the electron dot diagram for the following elements. �Calcium �Arsenic